Effectiveness of generic and proprietary first-line anti-retroviral regimens in a primary health care setting in Lusaka, Zambia: a cohort study
- PMID: 22493326
- PMCID: PMC3324461
- DOI: 10.1093/ije/dys022
Effectiveness of generic and proprietary first-line anti-retroviral regimens in a primary health care setting in Lusaka, Zambia: a cohort study
Abstract
Background: Although generic anti-retroviral drugs are in common use throughout the developing world, studies comparing their clinical effectiveness with that of proprietary formulations are lacking.
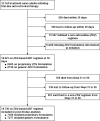
Methods: We analysed observational data from a large cohort of adults on anti-retroviral therapy (ART) to assess potential differences between generic and proprietary zidovudine (ZDV) formulations in post-90-day mortality, 'programme failure' (a composite of death, follow-up losses and withdrawals) and other clinical outcomes. We accounted for drug exposure in three ways: an 'initial dispensation' approach that categorized patients according to the first prescription; 'time-varying' approach that attributed an outcome to the formulation taken at the time of event; and 'predominant exposure' approach that considered only those with >75% exposure to either brand or generic ZDV. Proprietary formulations were used as the reference group in all adjusted Cox proportional hazard regressions.
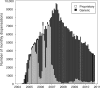
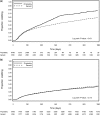
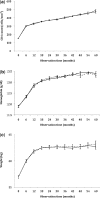
Results: Among 14 736 patients eligible for analysis, 7277 (49%) initiated a generic formulation of ZDV and 7459 (51%) initiated a proprietary formulation. When categorized according to initial dispensation, no difference in post-90-day mortality was observed between the two groups [adjusted hazard ratio (AHR): 0.93, 95% confidence interval (CI): 0.77-1.12]. Similar findings were noted when drug formulation was treated as a time-varying exposure (AHR: 1.15, 95% CI: 0.89-1.48) when analysis was limited to those with a predominant exposure to one formulation or the other (AHR: 0.59, 95% CI: 0.24-1.49). Results were consistent across all approaches when programme failure was considered as an outcome. No longitudinal differences were detected between formulations for CD4 response, weight change and haemoglobin concentration. Generic ZDV formulations were associated with slight decreases in single-drug substitution.
Conclusions: In this large programmatic cohort of adults starting ZDV-based first-line therapy, clinical outcomes appeared similar among patients on generic or proprietary formulations. These findings support continued use of generic anti-retroviral drug formulations in resource-constrained settings.
Figures
Comment in
-
Commentary: the past, present and future of affordable antiretroviral therapy in Africa.Int J Epidemiol. 2012 Apr;41(2):460-1. doi: 10.1093/ije/dys032. Int J Epidemiol. 2012. PMID: 22493327 No abstract available.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva: World Health Organization; 2010.
-
- Pinheiro E, Vasan A, Kim JY, Lee E, Guimier JM, Perriens J. Examining the production costs of antiretroviral drugs. AIDS. 2006;20:1745–52. - PubMed
-
- Kumarasamy N. Generic antiretroviral drugs—will they be the answer to HIV in the developing world? Lancet. 2004;364:3–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

