Diphenylarsinic acid promotes degradation of glutaminase C by mitochondrial Lon protease
- PMID: 22493432
- PMCID: PMC3365764
- DOI: 10.1074/jbc.M112.362699
Diphenylarsinic acid promotes degradation of glutaminase C by mitochondrial Lon protease
Abstract
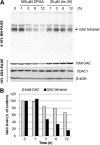
Glutaminase C (GAC), a splicing variant of the kidney-type glutaminase (KGA) gene, is a vital mitochondrial enzyme protein that catalyzes glutamine to glutamate. Earlier studies have shown that GAC proteins in the human hepatocarcinoma cell line, HepG2, were down-regulated by diphenylarsinic acid (DPAA), but the mechanism by which DPAA induced GAC protein down-regulation remained poorly understood. Here, we showed that DPAA promoted GAC protein degradation without affecting GAC transcription and translation. Moreover, DPAA-induced GAC proteolysis was mediated by mitochondrial Lon protease. DPAA insolubilized 0.5% Triton X-100-soluble GAC protein and promoted the accumulation of insoluble GAC in Lon protease knockdown cells. DPAA destroyed the native tetrameric GAC conformation and promoted an increase in the unassembled form of GAC when DPAA was incubated with cell extracts. Decreases in the tetrameric form of GAC were observed in cells exposed to DPAA, and decreases occurred prior to a decrease in total GAC protein levels. In addition, decreases in the tetrameric form of GAC were observed independently with Lon protease. Mitochondrial heat shock protein 70 is known to be an indispensable protein that can bind to misfolded proteins, thereby supporting degradation of proteins sensitive to Lon protease. When cells were incubated with DPAA, GAC proteins that can bind with mtHsp70 increased. Interestingly, the association of mtHsp70 with GAC protein increased when the tetrameric form of GAC was reduced. These results suggest that degradation of native tetrameric GAC by DPAA may be a trigger in GAC protein degradation by Lon protease.
Figures


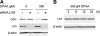






References
-
- Ishii K., Tamaoka A., Otsuka F., Iwasaki N., Shin K., Matsui A., Endo G., Kumagai Y., Ishii T., Shoji S., Ogata T., Ishizaki M., Doi M., Shimojo N. (2004) Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan. Ann. Neurol. 56, 741–745 - PubMed
-
- Kita K., Suzuki T., Ochi T. (2007) Down-regulation of glutaminase C in human hepatocarcinoma cell by diphenylarsinic acid, a degradation product of chemical warfare agents. Toxicol. Appl. Pharmacol. 220, 262–270 - PubMed
-
- Curthoys N. P., Watford M. (1995) Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 15, 133–159 - PubMed
-
- Elgadi K. M., Meguid R. A., Qian M., Souba W. W., Abcouwer S. F. (1999) Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genomics 1, 51–62 - PubMed
-
- Holcomb T., Taylor L., Trohkimoinen J., Curthoys N. P. (2000) Isolation, characterization, and expression of a human brain mitochondrial glutaminase cDNA. Mol. Brain Res. 76, 56–63 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

