α6β4 integrin, a master regulator of expression of integrins in human keratinocytes
- PMID: 22493440
- PMCID: PMC3365734
- DOI: 10.1074/jbc.M111.310458
α6β4 integrin, a master regulator of expression of integrins in human keratinocytes
Abstract
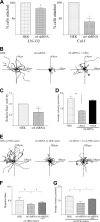
Three major laminin and collagen-binding integrins in skin (α6β4, α3β1, and α2β1) are involved in keratinocyte adhesion to the dermis and dissemination of skin cells during wound healing and/or tumorigenesis. Knockdown of α6 integrin in keratinocytes not only results in motility defects but also leads to decreased surface expression of the α2, α3, and β4 integrin subunits. Whereas α2 integrin mRNA levels are decreased in α6 integrin knockdown cells, α3 and β4 integrin mRNAs levels are unaffected. Expression of either α6 or α3 integrin in α6 integrin knockdown cells restores α2 integrin mRNA levels. Moreover, re-expression of α6 integrin increases β4 integrin protein at the cell surface, which results in an increase in α3 integrin expression via activation of initiation factor 4E-binding protein 1. Our data indicate that the α6β4 integrin is a master regulator of transcription and translation of other integrin subunits and underscore its pivotal role in wound healing and cancer.
Figures




References
-
- DiPersio C. M., van der Neut R., Georges-Labouesse E., Kreidberg J. A., Sonnenberg A., Hynes R. O. (2000) α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci. 113, 3051–3062 - PubMed
-
- Georges-Labouesse E., Messaddeq N., Yehia G., Cadalbert L., Dierich A., Le Meur M. (1996) Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370–373 - PubMed
-
- van der Neut R., Krimpenfort P., Calafat J., Niessen C. M., Sonnenberg A. (1996) Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 13, 366–369 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

