The cytoskeletal protein α-catenin unfurls upon binding to vinculin
- PMID: 22493458
- PMCID: PMC3365723
- DOI: 10.1074/jbc.M112.351023
The cytoskeletal protein α-catenin unfurls upon binding to vinculin
Abstract
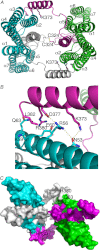
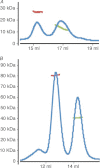
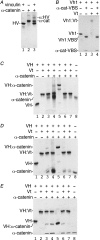
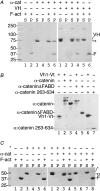
Adherens junctions (AJs) are essential for cell-cell contacts, morphogenesis, and the development of all higher eukaryotes. AJs are formed by calcium-dependent homotypic interactions of the ectodomains of single membrane-pass cadherin family receptors. These homotypic interactions in turn promote binding of the intracellular cytoplasmic tail domains of cadherin receptors with β-catenin, a multifunctional protein that plays roles in both transcription and AJs. The cadherin receptor-β-catenin complex binds to the cytoskeletal protein α-catenin, which is essential for both the formation and the stabilization of these junctions. Precisely how α-catenin contributes to the formation and stabilization of AJs is hotly debated, although the latter is thought to involve its interactions with the cytoskeletal protein vinculin. Here we report the crystal structure of the vinculin binding domain (VBD) of α-catenin in complex with the vinculin head domain (Vh1). This structure reveals that α-catenin is in a unique unfurled mode allowing dimer formation when bound to vinculin. Finally, binding studies suggest that vinculin must be in an activated state to bind to α-catenin and that this interaction is stabilized by the formation of a ternary α-catenin-vinculin-F-actin complex, which can be formed via the F-actin binding domain of either protein. We propose a feed-forward model whereby α-catenin-vinculin interactions promote their binding to the actin cytoskeleton to stabilize AJs.
Figures

References
-
- Braga V. M., Balda M. S. (2004) Regulation of cell-cell adhesion. Semin. Cell Dev. Biol. 15, 631–632 - PubMed
-
- Pokutta S., Weis W. I. (2002) The cytoplasmic face of cell contact sites. Curr. Opin. Struct. Biol. 12, 255–262 - PubMed
-
- Clevers H. (2006) Wnt/β-Catenin signaling in development and disease. Cell 127, 469–480 - PubMed
-
- Wahl J. K., 3rd, Kim Y. J., Cullen J. M., Johnson K. R., Wheelock M. J. (2003) N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J. Biol. Chem. 278, 17269–17276 - PubMed
-
- Haegel H., Larue L., Ohsugi M., Fedorov L., Herrenknecht K., Kemler R. (1995) Lack of β-catenin affects mouse development at gastrulation. Development 121, 3529–3537 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

