Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies
- PMID: 22493463
- PMCID: PMC3360917
- DOI: 10.1001/archinternmed.2012.332
Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies
Abstract
Background: Despite evidence that several colorectal cancer (CRC) screening strategies can reduce CRC mortality, screening rates remain low. This study aimed to determine whether the approach by which screening is recommended influences adherence.
Methods: We used a cluster randomization design with clinic time block as the unit of randomization. Persons at average risk for development of CRC in a racially/ethnically diverse urban setting were randomized to receive recommendation for screening by fecal occult blood testing (FOBT), colonoscopy, or their choice of FOBT or colonoscopy. The primary outcome was completion of CRC screening within 12 months after enrollment, defined as performance of colonoscopy, or 3 FOBT cards plus colonoscopy for any positive FOBT result. Secondary analyses evaluated sociodemographic factors associated with completion of screening.
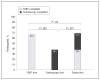
Results: A total of 997 participants were enrolled; 58% completed the CRC screening strategy they were assigned or chose. However, participants who were recommended colonoscopy completed screening at a significantly lower rate (38%) than participants who were recommended FOBT (67%) (P < .001) or given a choice between FOBT or colonoscopy (69%) (P < .001). Latinos and Asians (primarily Chinese) completed screening more often than African Americans. Moreover, nonwhite participants adhered more often to FOBT, while white participants adhered more often to colonoscopy.
Conclusions: The common practice of universally recommending colonoscopy may reduce adherence to CRC screening, especially among racial/ethnic minorities. Significant variation in overall and strategy-specific adherence exists between racial/ethnic groups; however, this may be a proxy for health beliefs and/or language. These results suggest that patient preferences should be considered when making CRC screening recommendations. Trial Registration clinicaltrials.gov Identifier: NCT00705731.
Figures

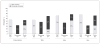
Comment in
-
The importance of choosing colorectal cancer screening tests: comment on "Adherence to colorectal cancer screening".Arch Intern Med. 2012 Apr 9;172(7):582-3. doi: 10.1001/archinternmed.2012.349. Arch Intern Med. 2012. PMID: 22493464 No abstract available.
References
-
- Wilson JMG, Jungner F. Principles and Practice of Screening for Disease. Geneva, Switzerland: World Health Organization; 1968.
-
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. - PubMed
-
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. - PubMed
-
- Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

