Roles for peroxisome proliferator-activated receptor γ (PPARγ) and PPARγ coactivators 1α and 1β in regulating response of white and brown adipocytes to hypoxia
- PMID: 22493496
- PMCID: PMC3365780
- DOI: 10.1074/jbc.M112.350918
Roles for peroxisome proliferator-activated receptor γ (PPARγ) and PPARγ coactivators 1α and 1β in regulating response of white and brown adipocytes to hypoxia
Abstract
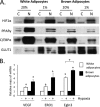
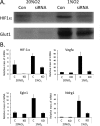
Obese white adipose tissue is hypoxic but is incapable of inducing compensatory angiogenesis. Brown adipose tissue is highly vascularized, facilitating delivery of nutrients to brown adipocytes for heat production. In this study, we investigated the mechanisms by which white and brown adipocytes respond to hypoxia. Brown adipocytes produced lower amounts of hypoxia-inducible factor 1α (HIF-1α) than white adipocytes in response to low O(2) but induced higher levels of hypoxia-associated genes. The response of white adipocytes to hypoxia required HIF-1α, but its presence alone was incapable of inducing target gene expression under normoxic conditions. In addition to the HIF-1α targets, hypoxia also induced many inflammatory genes. Exposure of white adipocytes to a peroxisome proliferator-activated receptor γ (PPARγ) ligand (troglitazone) attenuated induction of these genes but enhanced expression of the HIF-1α targets. Knockdown of PPARγ in mature white adipocytes prevented the usual robust induction of HIF-1α targets in response to hypoxia. Similarly, knockdown of PPARγ coactivator (PGC) 1β in PGC-1α-deficient brown adipocytes eliminated their response to hypoxia. These data demonstrate that the response of white adipocytes requires HIF-1α but also depends on PPARγ in white cells and the PPARγ cofactors PGC-1α and PGC-1β in brown cells.
Figures




References
-
- Halberg N., Khan T., Trujillo M. E., Wernstedt-Asterholm I., Attie A. D., Sherwani S., Wang Z. V., Landskroner-Eiger S., Dineen S., Magalang U. J., Brekken R. A., Scherer P. E. (2009) Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29, 4467–4483 - PMC - PubMed
-
- Pasarica M., Sereda O. R., Redman L. M., Albarado D. C., Hymel D. T., Roan L. E., Rood J. C., Burk D. H., Smith S. R. (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 - PMC - PubMed
-
- Trayhurn P., Wang B., Wood I. S. (2008) Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J. Nutr. 100, 227–235 - PubMed
-
- Wood I. S., de Heredia F. P., Wang B., Trayhurn P. (2009) Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc. 68, 370–377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

