Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9)
- PMID: 22493497
- PMCID: PMC3365958
- DOI: 10.1074/jbc.M112.363382
Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9)
Abstract
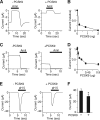
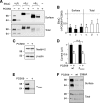
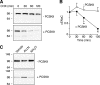
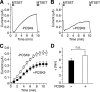
The epithelial Na(+) channel (ENaC) is critical for Na(+) homeostasis and blood pressure control. Defects in its regulation cause inherited forms of hypertension and hypotension. Previous work found that ENaC gating is regulated by proteases through cleavage of the extracellular domains of the α and γ subunits. Here we tested the hypothesis that ENaC is regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9), a protease that modulates the risk of cardiovascular disease. PCSK9 reduced ENaC current in Xenopus oocytes and in epithelia. This occurred through a decrease in ENaC protein at the cell surface and in the total cellular pool, an effect that did not require the catalytic activity of PCSK9. PCSK9 interacted with all three ENaC subunits and decreased their trafficking to the cell surface by increasing proteasomal degradation. In contrast to its previously reported effects on the LDL receptor, PCSK9 did not alter ENaC endocytosis or degradation of the pool of ENaC at the cell surface. These results support a role for PCSK9 in the regulation of ENaC trafficking in the biosynthetic pathway, likely by increasing endoplasmic reticulum-associated degradation. By reducing ENaC channel number, PCSK9 could modulate epithelial Na(+) absorption, a major contributor to blood pressure control.
Figures


Similar articles
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment.Proc Natl Acad Sci U S A. 2005 Feb 8;102(6):2069-74. doi: 10.1073/pnas.0409736102. Epub 2005 Jan 27. Proc Natl Acad Sci U S A. 2005. PMID: 15677715 Free PMC article.
-
Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels.J Biol Chem. 2012 Dec 21;287(52):43482-91. doi: 10.1074/jbc.M112.380618. Epub 2012 Nov 7. J Biol Chem. 2012. PMID: 23135270 Free PMC article.
-
Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR.J Lipid Res. 2012 Sep;53(9):1932-43. doi: 10.1194/jlr.M028563. Epub 2012 Jul 4. J Lipid Res. 2012. PMID: 22764087 Free PMC article.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9): lessons learned from patients with hypercholesterolemia.Clin Chem. 2014 Nov;60(11):1380-9. doi: 10.1373/clinchem.2014.225946. Epub 2014 Sep 23. Clin Chem. 2014. PMID: 25248569 Review.
Cited by
-
Potential Link Between Proprotein Convertase Subtilisin/Kexin Type 9 and Alzheimer's Disease.Int J Biomed Investig. 2018;1(1):106. doi: 10.31531/2581-4745.1000106. Epub 2018 Mar 27. Int J Biomed Investig. 2018. PMID: 32352077 Free PMC article.
-
Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs.Curr Atheroscler Rep. 2014 Sep;16(9):439. doi: 10.1007/s11883-014-0439-8. Curr Atheroscler Rep. 2014. PMID: 25052769 Review.
-
Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside.Signal Transduct Target Ther. 2024 Jan 8;9(1):13. doi: 10.1038/s41392-023-01690-3. Signal Transduct Target Ther. 2024. PMID: 38185721 Free PMC article. Review.
-
PCSK9: From Basic Science Discoveries to Clinical Trials.Circ Res. 2018 May 11;122(10):1420-1438. doi: 10.1161/CIRCRESAHA.118.311227. Circ Res. 2018. PMID: 29748367 Free PMC article. Review.
-
Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way.Basic Res Cardiol. 2017 Sep 14;112(6):63. doi: 10.1007/s00395-017-0650-1. Basic Res Cardiol. 2017. PMID: 28913715 Free PMC article.
References
-
- Schild L. (2004) The epithelial sodium channel. From molecule to disease. Rev. Physiol. Biochem. Pharmacol. 151, 93–107 - PubMed
-
- Snyder P. M. (2005) Minireview. Regulation of epithelial Na+ channel trafficking. Endocrinology 146, 5079–5085 - PubMed
-
- Snyder P. M., Price M. P., McDonald F. J., Adams C. M., Volk K. A., Zeiher B. G., Stokes J. B., Welsh M. J. (1995) Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83, 969–978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

