The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens
- PMID: 22493504
- PMCID: PMC3365761
- DOI: 10.1074/jbc.M111.336073
The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens
Abstract
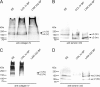
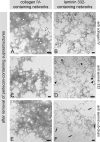
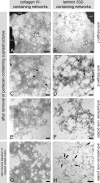
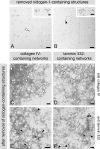
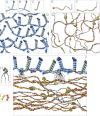
The basement membrane between the epidermis and the dermis is indispensable for normal skin functions. It connects, and functionally separates, the epidermis and the dermis. To understand the suprastructural and functional basis of these connections, heterotypic supramolecular aggregates were isolated from the dermal-epidermal junction zone of human skin. Individual suprastructures were separated and purified by immunomagnetic beads, each recognizing a specific, molecular component of the aggregates. The molecular compositions of the suprastructures were determined by immunogold electron microscopy and immunoblotting. A composite of two networks was obtained from fibril-free suspensions by immunobeads recognizing either laminin 332 or collagen IV. After removal of perlecan-containing suprastructures or after enzyme digestion of heparan sulfate chains, a distinct network with a diffuse electron-optical appearance was isolated with magnetic beads coated with antibodies to collagen IV. The second network was more finely grained and comprised laminin 332 and laminins with α5-chains. The core protein of perlecan was an exclusive component of this network whereas its heparan sulfate chains were integrated into the collagen IV-containing network. Nidogens 1 and 2 occurred in both networks but did not form strong molecular cross-bridges. Their incorporation into one network appeared to be masked after their incorporation into the other one. We conclude that the epidermal basement membrane is a composite of two structurally independent networks that are tightly connected in a spot-welding-like manner by perlecan-containing aggregates.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

