Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer
- PMID: 22493549
- PMCID: PMC3319962
- DOI: 10.3748/wjg.v18.i12.1357
Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer
Abstract
Aim: To investigate second-line chemotherapy in gemcitabine-pretreated patients with advanced or metastatic pancreatic cancer [(frequency, response, outcome, course of carbohydrate antigen 19-9 (CA 19-9)].
Methods: This retrospective study included all patients with advanced or metastatic pancreatic cancer (adenocarcinoma or carcinoma) treated with second-line chemotherapy in our center between 2000 and 2008. All patients received first-line chemotherapy with gemcitabine, and prior surgery or radiotherapy was permitted. We analyzed each chemotherapy protocol for second-line treatment, the number of cycles and the type of combination used. The primary endpoint was overall survival. Secondary endpoints included progression-free survival, response rate, grade 3-4 toxicity, dosage modifications and CA 19-9 course.
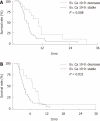
Results: A total of eighty patients (38%) underwent a second-line therapy among 206 patients who had initially received first-line treatment with a gemcitabine-based regimen. Median number of cycles was 4 (range: 1-12) and the median duration of treatment was 2.6 mo (range: 0.3-7.4). The overall disease control rate was 40.0%. The median overall survival and progression-free survival from the start of second-line therapy were 5.8 (95% CI: 4.1-6.6) and 3.4 mo (95% CI: 2.4-4.2), respectively. Toxicity was generally acceptable. Median overall survival of patients with a CA 19-9 level declining by more than 20% was 10.3 mo (95% CI: 4.5-11.6) vs 5.2 mo (95% CI: 4.0-6.4) for others (P = 0.008).
Conclusion: A large proportion of patients could benefit from second-line therapy, and CA 19-9 allows efficient treatment monitoring both in first and second-line chemotherapy.
Keywords: Carbohydrate antigen 19-9; Chemotherapy; Gemcitabine; Pancreatic cancer; Second-line.
Figures
References
-
- National Cancer Institute, Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Pancreas. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html.
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical