Event timing in associative learning: from biochemical reaction dynamics to behavioural observations
- PMID: 22493657
- PMCID: PMC3316544
- DOI: 10.1371/journal.pone.0032885
Event timing in associative learning: from biochemical reaction dynamics to behavioural observations
Abstract
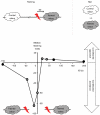
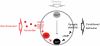
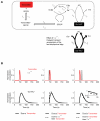
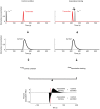
Associative learning relies on event timing. Fruit flies for example, once trained with an odour that precedes electric shock, subsequently avoid this odour (punishment learning); if, on the other hand the odour follows the shock during training, it is approached later on (relief learning). During training, an odour-induced Ca(++) signal and a shock-induced dopaminergic signal converge in the Kenyon cells, synergistically activating a Ca(++)-calmodulin-sensitive adenylate cyclase, which likely leads to the synaptic plasticity underlying the conditioned avoidance of the odour. In Aplysia, the effect of serotonin on the corresponding adenylate cyclase is bi-directionally modulated by Ca(++), depending on the relative timing of the two inputs. Using a computational approach, we quantitatively explore this biochemical property of the adenylate cyclase and show that it can generate the effect of event timing on associative learning. We overcome the shortage of behavioural data in Aplysia and biochemical data in Drosophila by combining findings from both systems.
Conflict of interest statement
Figures

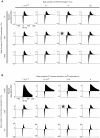
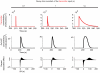
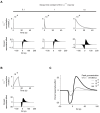

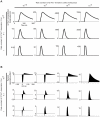
References
-
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. - PubMed
-
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. - PubMed
-
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information Processing in Animals: Memory Mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47.
-
- Sutton RS, Barto AG. Time-derivative models of pavlovian reinforcement. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: MIT Press; 1990. pp. 497–537.
-
- Chang RC, Blaisdell AP, Miller RR. Backward conditioning: mediation by the context. J Exp Psychol Anim Behav Process. 2003;29:171–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

