Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis
- PMID: 22493705
- PMCID: PMC3321026
- DOI: 10.1371/journal.pone.0034647
Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis
Abstract
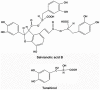
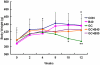
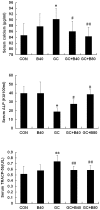
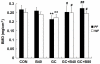
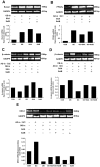
Glucocorticoid (GC) induced osteoporosis (GIO) is caused by the long-term use of GC for treatment of autoimmune and inflammatory diseases. The GC related disruption of bone marrow microcirculation and increased adipogenesis contribute to GIO development. However, neither currently available anti-osteoporosis agent is completely addressed to microcirculation and bone marrow adipogenesis. Salvianolic acid B (Sal B) is a polyphenolic compound from a Chinese herbal medicine, Salvia miltiorrhiza Bunge. The aim of this study was to determine the effects of Sal B on osteoblast bone formation, angiogenesis and adipogenesis-associated GIO by performing marrow adipogenesis and microcirculation dilation and bone histomorphometry analyses. (1) In vivo study: Bone loss in GC treated rats was confirmed by significantly decreased BMD, bone strength, cancellous bone mass and architecture, osteoblast distribution, bone formation, marrow microvessel density and diameter along with down-regulation of marrow BMPs expression and increased adipogenesis. Daily treatment with Sal B (40 mg/kg/d) for 12 weeks in GC male rats prevented GC-induced cancellous bone loss and increased adipogenesis while increasing cancellous bone formation rate with improved local microcirculation by capillary dilation. Treatment with Sal B at a higher dose (80 mg/kg/d) not only prevented GC-induced osteopenia, but also increased cancellous bone mass and thickness, associated with increase of marrow BMPs expression, inhibited adipogenesis and further increased microvessel diameters. (2) In vitro study: In concentration from 10(-6) mol/L to 10(-7) mol/L, Sal B stimulated bone marrow stromal cell (MSC) differentiation to osteoblast and increased osteoblast activities, decreased GC associated adipogenic differentiation by down-regulation of PPARγ mRNA expression, increased Runx2 mRNA expression without osteoblast inducement, and, furthermore, Sal B decreased Dickkopf-1 and increased β-catenin mRNA expression with or without adipocyte inducement in MSC. We conclude that Sal B prevented bone loss in GC-treated rats through stimulation of osteogenesis, bone marrow angiogenesis and inhibition of adipogenesis.
Conflict of interest statement
Figures







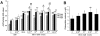
References
-
- Shah SK, Gecys GT. Prednisone-Induced Osteoporosis: An Overlooked and Undertreated Adverse Effect. J Am Osteopath Assoc. 2006;106:653–657. - PubMed
-
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102. - PubMed
-
- Ito S, Suzuki N, Kato S. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone. 2007;40:84–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

