Repertoire enhancement with adoptively transferred female lymphocytes controls the growth of pre-implanted murine prostate cancer
- PMID: 22493742
- PMCID: PMC3320876
- DOI: 10.1371/journal.pone.0035222
Repertoire enhancement with adoptively transferred female lymphocytes controls the growth of pre-implanted murine prostate cancer
Abstract
Background: In prostate cancer, genes encoding androgen-regulated, Y-chromosome-encoded, and tissue-specific antigens may all be overexpressed. In the adult male host, however, most high affinity T cells targeting these potential tumor rejection antigens will be removed during negative selection. In contrast, the female mature T-cell repertoire should contain abundant precursors capable of recognizing these classes of prostate cancer antigens and mediating effective anti-tumor immune responses.
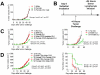
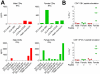
Methodology/principal findings: We find that syngeneic TRAMP-C2 prostatic adenocarcinoma cells are spontaneously rejected in female hosts. Adoptive transfer of naïve female lymphocytes to irradiated male hosts bearing pre-implanted TRAMP-C2 tumor cells slows tumor growth and mediates tumor rejection in some animals. The success of this adoptive transfer was dependent on the transfer of female CD4 T cells and independent of the presence of CD25-expressing regulatory T cells in the transferred lymphocytes. We identify in female CD4 T cells stimulated with TRAMP-C2 a dominant MHC II-restricted response to the Y-chromosome antigen DBY. Furthermore, CD8 T cell responses in female lymphocytes to the immunodominant MHC I-restricted antigen SPAS-1 are markedly increased compared to male mice. Finally, we find no exacerbation of graft-versus-host disease in either syngeneic or minor-antigen mismatched allogeneic lymphocyte adoptive transfer models by using female into male versus male into male cells.
Conclusions/significance: This study shows that adoptively transferred female lymphocytes, particularly CD4 T cells, can control the outgrowth of pre-implanted prostatic adenocarcinoma cells. This approach does not significantly worsen graft-versus-host responses suggesting it may be viable in the clinic. Further, enhancing the available immune repertoire with female-derived T cells may provide an excellent pool of prostate cancer reactive T cells for further augmentation by combination with either vaccination or immune regulatory blockade strategies.
Conflict of interest statement
Figures
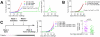

References
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. - PubMed
-
- Xu LL, Su YP, Labiche R, Segawa T, Shanmugam N, et al. Quantitative expression profile of androgen-regulated genes in prostate cancer cells and identification of prostate-specific genes. Int J Cancer. 2001;92:322–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

