Growth hormone secretagogues protect mouse cardiomyocytes from in vitro ischemia/reperfusion injury through regulation of intracellular calcium
- PMID: 22493744
- PMCID: PMC3320867
- DOI: 10.1371/journal.pone.0035265
Growth hormone secretagogues protect mouse cardiomyocytes from in vitro ischemia/reperfusion injury through regulation of intracellular calcium
Abstract
Background: Ischemic heart disease is a leading cause of mortality. To study this disease, ischemia/reperfusion (I/R) models are widely used to mimic the process of transient blockage and subsequent recovery of cardiac coronary blood supply. We aimed to determine whether the presence of the growth hormone secretagogues, ghrelin and hexarelin, would protect/improve the function of heart from I/R injury and to examine the underlying mechanisms.
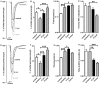
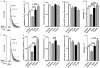
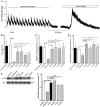
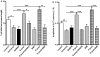
Methodology/principal findings: Isolated hearts from adult male mice underwent 20 min global ischemia and 30 min reperfusion using a Langendorff apparatus. Ghrelin (10 nM) or hexarelin (1 nM) was introduced into the perfusion system either 10 min before or after ischemia, termed pre- and post-treatments. In freshly isolated cardiomyocytes from these hearts, single cell shortening, intracellular calcium ([Ca(2+)](i)) transients and caffeine-releasable sarcoplasmic reticulum (SR) Ca(2+) were measured. In addition, RT-PCR and Western blots were used to examine the expression level of GHS receptor type 1a (GHS-R1a), and phosphorylated phospholamban (p-PLB), respectively. Ghrelin and hexarelin pre- or post-treatments prevented the significant reduction in the cell shortening, [Ca(2+)](i) transient amplitude and caffeine-releasable SR Ca(2+) content after I/R through recovery of p-PLB. GHS-R1a antagonists, [D-Lys3]-GHRP-6 (200 nM) and BIM28163 (100 nM), completely blocked the effects of GHS on both cell shortening and [Ca(2+)](i) transients.
Conclusion/significance: Through activation of GHS-R1a, ghrelin and hexarelin produced a positive inotropic effect on ischemic cardiomyocytes and protected them from I/R injury probably by protecting or recovering p-PLB (and therefore SR Ca(2+) content) to allow the maintenance or recovery of normal cardiac contractility. These observations provide supporting evidence for the potential therapeutic application of ghrelin and hexarelin in patients with cardiac I/R injury.
Conflict of interest statement
Figures

References
-
- Ostadal B, Kolář F. Cardiac ischemia: from injury to protection: Springer. 1999.
-
- Headrick JP, Peart J, Hack B, Flood A, Matherne GP. Functional properties and responses to ischaemia-reperfusion in Langendorff perfused mouse heart. Exp Physiol. 2001;86:703–716. - PubMed
-
- Furman E, Acad BA, Sonn J, Raul A, Kedem J. Effect of global vs regional ischaemia upon myocardial contractility and oxygen balance. Cardiovasc Res. 1985;19:606–612. - PubMed
-
- Chambers DJ, Fallouh HB. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol Ther. 2010;127:41–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

