Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury
- PMID: 22494498
- PMCID: PMC3350422
- DOI: 10.1186/1471-2377-12-20
Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury
Abstract
Background: The α2-adrenoreceptor agonist dexmedetomidine is known to provide neuroprotection under ischemic conditions. In this study we investigated whether dexmedetomidine has a protective effect in an in vitro model for traumatic brain injury.
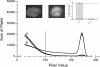
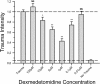
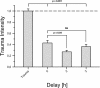
Methods: Organotypic hippocampal slice cultures were subjected to a focal mechanical trauma and then exposed to varying concentrations of dexmedetomidine. After 72 h cell injury was assessed using propidium iodide. In addition, the effects of delayed dexmedetomidine application, of hypothermia and canonical signalling pathway inhibitors were examined.
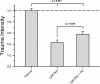
Results: Dexmedetomidine showed a protective effect on traumatically injured hippocampal cells with a maximum effect at a dosage of 1 μM. This effect was partially reversed by the simultaneous administration of the ERK inhibitor PD98059.
Conclusion: In this TBI model dexmedetomidine had a significant neuroprotective effect. Our results indicate that activation of ERK might be involved in mediating this effect.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

