Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics
- PMID: 22494889
- PMCID: PMC3366278
- DOI: 10.1016/j.biomaterials.2012.03.065
Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics
Abstract
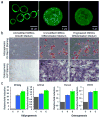
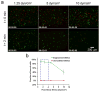
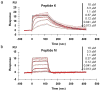
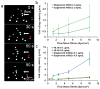
Dynamic cell-microenvironment interactions regulate many biological events and play a critical role in tissue regeneration. Cell homing to targeted tissues requires well balanced interactions between cells and adhesion molecules on blood vessel walls. However, many stem cells lack affinity with adhesion molecules. It is challenging and clinically important to engineer these stem cells to modulate their dynamic interactions with blood vessels. In this study, a new chemical strategy was developed to engineer cell-microenvironment interactions. This method allowed the conjugation of peptides onto stem cell membranes without affecting cell viability, proliferation or multipotency. Mesenchymal stem cells (MSCs) engineered in this manner showed controlled firm adhesion and rolling on E-selectin under physiological shear stresses. For the first time, these biomechanical responses were achieved by tuning the binding kinetics of the peptide-selectin interaction. Rolling of engineered MSCs on E-selectin is mediated by a Ca(2+) independent interaction, a mechanism that differs from the Ca(2+) dependent physiological process. This further illustrates the ability of this approach to manipulate cell-microenvironment interactions, in particular for the application of delivering cells to targeted tissues. It also provides a new platform to engineer cells with multiple functionalities.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Mahal LK, Bertozzi CR. Engineered cell surfaces: Fertile ground for molecular landscaping. Chem Biol. 1997;4(6):415–422. - PubMed
-
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14(2):181–187. - PubMed
-
- Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat Nanotechnol. 2008;3(1):36–40. - PubMed
-
- Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Sortagging: a versatile method for protein labeling. Nat Chem Biol. 2007;3(11):707–708. - PubMed
-
- Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20(5):531–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

