Serotonin inhibits low-threshold spike interneurons in the striatum
- PMID: 22495583
- PMCID: PMC3424750
- DOI: 10.1113/jphysiol.2011.219469
Serotonin inhibits low-threshold spike interneurons in the striatum
Abstract
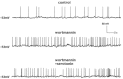
Low-threshold spike interneurons (LTSIs) are important elements of the striatal architecture and the only known source of nitric oxide in this nucleus, but their rarity has so far prevented systematic studies. Here, we used transgenic mice in which green fluorescent protein is expressed under control of the neuropeptide Y (NPY) promoter and striatal NPY-expressing LTSIs can be easily identified, to investigate the effects of serotonin on these neurons. In sharp contrast with its excitatory action on other striatal interneurons, serotonin (30 μM) strongly inhibited LTSIs, reducing or abolishing their spontaneous firing activity and causing membrane hyperpolarisations.These hyperpolarisations persisted in the presence of tetrodotoxin, were mimicked by 5-HT(2C) receptor agonists and reversed by 5-HT(2C) antagonists. Voltage-clamp slow-ramp experiments showed that serotonin caused a strong increase in an outward current activated by depolarisations that was blocked by the specific M current blocker XE 991. In current-clamp experiments,XE 991 per se caused membrane depolarisations in LTSIs and subsequent application of serotonin (in the presence of XE 991) failed to affect these neurons.We concluded that serotonin strongly inhibits striatal LTSIs acting through postsynaptic 5-HT(2C) receptors and increasing an M type current.
Figures




References
-
- Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y-induced enhancement of the evoked release of newly synthesized dopamine in rat striatum: mediation by Y2 receptors. Neuropharmacology. 2007;52:1396–1402. - PubMed
-
- Bedard C, Wallman MJ, Pourcher E, Gould PV, Parent A, Parent M. Serotonin and dopamine striatal innervation in Parkinson's disease and Huntington's chorea. Parkinsonism Relat Disord. 2011;17:593–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

