Mechanical regulation of fibroblast migration and collagen remodelling in healing myocardial infarcts
- PMID: 22495588
- PMCID: PMC3477759
- DOI: 10.1113/jphysiol.2012.229484
Mechanical regulation of fibroblast migration and collagen remodelling in healing myocardial infarcts
Abstract
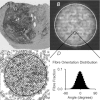
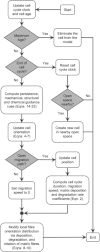
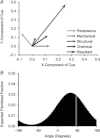
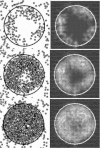
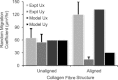
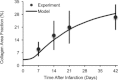
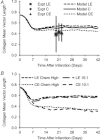
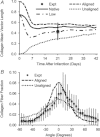
Effective management of healing and remodelling after myocardial infarction is an important problem in modern cardiology practice. We have recently shown that the level of infarct anisotropy is a critical determinant of heart function following a large anterior infarction, which suggests that therapeutic gains may be realized by controlling infarct anisotropy. However, factors regulating infarct anisotropy are not well understood. Mechanical, structural and chemical guidance cues have all been shown to regulate alignment of fibroblasts and collagen in vitro, and prior studies have proposed that each of these cues could regulate anisotropy of infarct scar tissue, but understanding of fibroblast behaviour in the complex environment of a healing infarct is lacking. We developed an agent-based model of infarct healing that accounted for the combined influence of these cues on fibroblast alignment, collagen deposition and collagen remodelling. We pooled published experimental data from several sources in order to determine parameter values, then used the model to test the importance of each cue for predicting collagen alignment measurements from a set of recent cryoinfarction experiments. We found that although chemokine gradients and pre-existing matrix structures had important effects on collagen organization, a response of fibroblasts to mechanical cues was critical for correctly predicting collagen alignment in infarct scar. Many proposed therapies for myocardial infarction, such as injection of cells or polymers, alter the mechanics of the infarct region. Our modelling results suggest that such therapies could change the anisotropy of the healing infarct, which could have important functional consequences. This model is therefore a potentially important tool for predicting how such interventions change healing outcomes.
Figures
References
-
- Bailón-Plaza A, van der Meulen MCH. A mathematical framework to study the effects of growth factor influences on fracture healing. J Theor Biol. 2001;212:191–209. - PubMed
-
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. - PubMed
-
- Chavali AK, Gianchandani EP, Tung KS, Lawrence MB, Peirce SM, Papin JA. Characterizing emergent properties of immunological systems with multi-cellular rule-based computational modeling. Trends Immunol. 2008;29:589–599. - PubMed
-
- Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

