Modelling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit
- PMID: 22495592
- PMCID: PMC3477749
- DOI: 10.1113/jphysiol.2012.227926
Modelling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit
Abstract
Triggered release of Ca2+ from an individual sarcoplasmic reticulum (SR) Ca(2+) release unit (CRU) is the fundamental event of cardiac excitation–contraction coupling, and spontaneous release events (sparks) are the major contributor to diastolic Ca(2+) leak in cardiomyocytes. Previous model studies have predicted that the duration and magnitude of the spark is determined by the local CRU geometry, as well as the localization and density of Ca(2+) handling proteins. We have created a detailed computational model of a CRU, and developed novel tools to generate the computational geometry from electron tomographic images. Ca(2+) diffusion was modelled within the SR and the cytosol to examine the effects of localization and density of the Na(+)/Ca(2+) exchanger, sarco/endoplasmic reticulum Ca(2+)-ATPase 2 (SERCA), and calsequestrin on spark dynamics. We reconcile previous model predictions of approximately 90% local Ca(2+) depletion in junctional SR, with experimental reports of about 40%. This analysis supports the hypothesis that dye kinetics and optical averaging effects can have a significant impact on measures of spark dynamics. Our model also predicts that distributing calsequestrin within non-junctional Z-disc SR compartments, in addition to the junctional compartment, prolongs spark release time as reported by Fluo5. By pumping Ca(2+) back into the SR during a release, SERCA is able to prolong a Ca(2+) spark, and this may contribute to SERCA-dependent changes in Ca(2+) wave speed. Finally, we show that including the Na(+)/Ca(2+) exchanger inside the dyadic cleft does not alter local [Ca(2+)] during a spark.
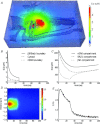
Figures





References
-
- Altamirano J, Bers DM. Effect of intracellular Ca2+ and action potential duration on L-type Ca2+ channel inactivation and recovery from inactivation in rabbit cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H563–H573. - PubMed
-
- Asghari P, Scriven DRL, Hoskins J, Fameli N, van Breemen C, Moore EDW. The structure and functioning of the couplon in the mammalian cardiomyocyte. Protoplasma. 2011;249(Suppl 1):S31–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P01HL46345/HL/NHLBI NIH HHS/United States
- 1R01 HL96544/HL/NHLBI NIH HHS/United States
- P41 GM103412/GM/NIGMS NIH HHS/United States
- R01 HL096544/HL/NHLBI NIH HHS/United States
- 1R01 GM31749/GM/NIGMS NIH HHS/United States
- R01 GM031749/GM/NIGMS NIH HHS/United States
- P41 GM103426/GM/NIGMS NIH HHS/United States
- 8P41GM103426-19/GM/NIGMS NIH HHS/United States
- RR004050/GM103412/GM/NIGMS NIH HHS/United States
- R01 HL105242/HL/NHLBI NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- P01 HL046345/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

