Watching tumours gasp and die with MRI: the promise of hyperpolarised 13C MR spectroscopic imaging
- PMID: 22496072
- PMCID: PMC3474112
- DOI: 10.1259/bjr/81120511
Watching tumours gasp and die with MRI: the promise of hyperpolarised 13C MR spectroscopic imaging
Abstract
A better understanding of tumour biology has led to the development of "targeted therapies", in which a drug is designed to disrupt a specific biochemical pathway important for tumour cell survival or proliferation. The introduction of these drugs into the clinic has shown that patients can vary widely in their responses. Molecular imaging is likely to play an increasingly important role in predicting and detecting these responses and thus in guiding treatment in individual patients: so-called "personalised medicine". The aim of this review is to discuss how hyperpolarised (13)C MR spectroscopic imaging might be used for treatment response monitoring. This technique, which increases the sensitivity of detection of injected (13)C-labelled molecules by >10,000-fold, has allowed a new approach to metabolic imaging. The basic principles of the technique and its potential advantages over other imaging methods for detecting early evidence of treatment response will be discussed. Given that the technique is poised to translate to the clinic, I will also speculate on its likely applications.
Figures
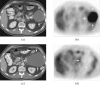


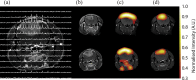
 into a tumour-bearing mouse. The image resolution was 16×16 voxels, each of which measured 2×2×6 mm. The images were smoothed by multiplying by a cosine function and zero filling to 128 points in both spatial directions, line broadening to 30 Hz and then zero filling to 512 points in the spectral direction before Fourier transformation.
into a tumour-bearing mouse. The image resolution was 16×16 voxels, each of which measured 2×2×6 mm. The images were smoothed by multiplying by a cosine function and zero filling to 128 points in both spatial directions, line broadening to 30 Hz and then zero filling to 512 points in the spectral direction before Fourier transformation.  and CO2 peaks were fitted in the frequency domain and only pixels with a frequency separation between the two peaks of 36±1 ppm were included. These 13C spectroscopic images are superimposed on a 1H image of tissue anatomy (i). The tumour margin is outlined in red in (i) and in white in (ii–iv). The ratio of the CO2 (iv) and
and CO2 peaks were fitted in the frequency domain and only pixels with a frequency separation between the two peaks of 36±1 ppm were included. These 13C spectroscopic images are superimposed on a 1H image of tissue anatomy (i). The tumour margin is outlined in red in (i) and in white in (ii–iv). The ratio of the CO2 (iv) and  (iii) images can be used to calculate an image of extracellular pH (ii). (b) 13C MR spectra acquired from tumours following intravenous injection of [1-13C]-ascorbic acid (i) and [1-13C]-dehydroascorbic acid (ii). Sequential spectra were collected over a period of 16 s (i) and 32 s (ii). While there was no observable flux of hyperpolarised 13C label from [1-13C]-ascorbic acid (179 ppm) to [1-13C]-dehydroascorbic acid (175 ppm) (i), significant label flux was observed from [1-13C]-dehydroascorbic acid to [1-13C]-ascorbic acid, indicating rapid reduction of the injected [1-13C]-dehydroascorbic acid in the tumour. Reproduced with permission from Gallagher et al [59] and Bohndiek et al [60].
(iii) images can be used to calculate an image of extracellular pH (ii). (b) 13C MR spectra acquired from tumours following intravenous injection of [1-13C]-ascorbic acid (i) and [1-13C]-dehydroascorbic acid (ii). Sequential spectra were collected over a period of 16 s (i) and 32 s (ii). While there was no observable flux of hyperpolarised 13C label from [1-13C]-ascorbic acid (179 ppm) to [1-13C]-dehydroascorbic acid (175 ppm) (i), significant label flux was observed from [1-13C]-dehydroascorbic acid to [1-13C]-ascorbic acid, indicating rapid reduction of the injected [1-13C]-dehydroascorbic acid in the tumour. Reproduced with permission from Gallagher et al [59] and Bohndiek et al [60].References
-
- Twombly R. Avastin's uncertain future in breast cancer treatment. J Natl Cancer Inst 103:458–60 - PubMed
-
- Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 2007;25:587–95 - PubMed
-
- Sawyers CL. The cancer biomarker problem. Nature 2008;452:548–52 - PubMed
-
- Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer 2008;8:94–107 - PubMed
-
- Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

