Sustained expression of osteopontin is closely associated with calcium deposits in the rat hippocampus after transient forebrain ischemia
- PMID: 22496158
- PMCID: PMC3460356
- DOI: 10.1369/0022155412441707
Sustained expression of osteopontin is closely associated with calcium deposits in the rat hippocampus after transient forebrain ischemia
Abstract
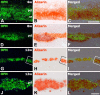
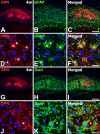
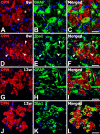
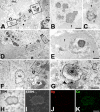
The present study was designed to evaluate the extent and topography of osteopontin (OPN) protein expression in the rat hippocampus 4 to 12 weeks following transient forebrain ischemia, and to compare OPN expression patterns with those of calcium deposits and astroglial and microglial reactions. Two patterns of OPN staining were recognized by light microscopy: 1) a diffuse pattern of tiny granular deposits throughout the CA1 region at 4 weeks after ischemia and 2) non-diffuse ovoid to round deposits, which formed conglomerates in the CA1 pyramidal cell layer over the chronic interval of 8 to 12 weeks. Immunogold-silver electron microscopy and electron probe microanalysis demonstrated that OPN deposits were indeed diverse types of calcium deposits, which were clearly delineated by profuse silver grains indicative of OPN expression. Intracellular OPN deposits were frequently observed within reactive astrocytes and neurons 4 weeks after ischemia but rarely at later times. By contrast, extracellular OPN deposits progressively increased in size and appeared to be gradually phagocytized by microglia or brain macrophages and some astrocytes over 8 to 12 weeks. These data indicate an interaction between OPN and calcium in the hippocampus in the chronic period after ischemia, suggesting that OPN binding to calcium deposits may be involved in scavenging mechanisms.
Conflict of interest statement
Figures


References
-
- Agnew WF, Yuen TG, Bullara LA, Jacques D, Pudenz RH. 1979. Intracellular calcium deposition in brain following electrical stimulation. Neurol Res. 1:187–202 - PubMed
-
- Bendel O, Bueters T, von Euler M, Ove Ogren S, Sandin J, von Euler G. 2005. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 25:1586–1595 - PubMed
-
- Bonnekoh P, Kuroiwa T, Kloiber O, Hossmann K. 1992. Time profile of calcium accumulation in hippocampus, striatum and frontoparietal cortex after transient forebrain ischemia in the gerbil. Acta Neuropathol. 84:400–406 - PubMed
-
- Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. 1993. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 22:147–159 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

