Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes
- PMID: 22496370
- PMCID: PMC3370237
- DOI: 10.1074/jbc.M111.323279
Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes
Abstract
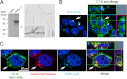
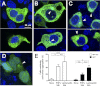
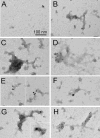
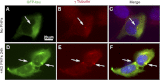
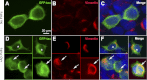
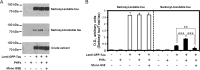
Abnormal folding of tau protein leads to the generation of paired helical filaments (PHFs) and neurofibrillary tangles, a key neuropathological feature in Alzheimer disease and tauopathies. A specific anatomical pattern of pathological changes developing in the brain suggests that once tau pathology is initiated it propagates between neighboring neuronal cells, possibly spreading along the axonal network. We studied whether PHFs released from degenerating neurons could be taken up by surrounding cells and promote spreading of tau pathology. Neuronal and non-neuronal cells overexpressing green fluorescent protein-tagged tau (GFP-Tau) were treated with isolated fractions of human Alzheimer disease-derived PHFs for 24 h. We found that cells internalized PHFs through an endocytic mechanism and developed intracellular GFP-Tau aggregates with attributes of aggresomes. This was particularly evident by the perinuclear localization of aggregates and redistribution of the vimentin intermediate filament network and retrograde motor protein dynein. Furthermore, the content of Sarkosyl-insoluble tau, a measure of abnormal tau aggregation, increased 3-fold in PHF-treated cells. An exosome-related mechanism did not appear to be involved in the release of GFP-Tau from untreated cells. The evidence that cells can internalize PHFs, leading to formation of aggresome-like bodies, opens new therapeutic avenues to prevent propagation and spreading of tau pathology.
Figures



References
-
- Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 - PubMed
-
- Avila J., Lucas J. J., Perez M., Hernandez F. (2004) Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 84, 361–384 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

