Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice
- PMID: 22496397
- PMCID: PMC3382842
- DOI: 10.1124/dmd.112.045112
Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice
Abstract
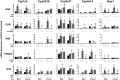
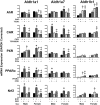
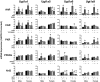
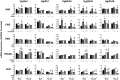
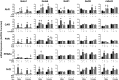
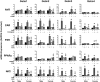
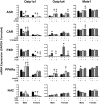
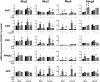
The transcription factors aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane X receptor (PXR), peroxisome proliferator-activated receptor α (PPARα), and nuclear factor erythroid 2-related factor 2 (Nrf2) regulate genes encoding drug-metabolizing enzymes and transporters in livers of mice after chemical activation. However, the specificity of their transcriptional regulation has not been determined systematically in vivo. The purpose of this study was to identify genes encoding drug-metabolizing enzymes and transporters altered by chemical activators in a transcription factor-dependent manner using wild-type and transcription factor-null mice. Chemical activators were administered intraperitoneally to mice once daily for 4 days. Livers were collected 24 h after the final dose, and total RNA was isolated for mRNA quantification of cytochromes P450, NAD(P)H quinone oxidoreductase 1 (Nqo1), aldehyde dehydrogenases (Aldhs), glutathione transferases (Gsts), sulfotransferases (Sults), UDP-glucuronosyltransferases (Ugts), organic anion-transporting polypeptides (Oatps), and multidrug resistance-associated proteins (Mrps). Pharmacological activation of each transcription factor leads to mRNA induction of drug metabolic and transport genes in livers of male and female wild-type mice, but no change in null mice: AhR (Cyp1a2, Nqo1, Aldh7a1, Ugt1a1, Ugt1a6, Ugt1a9, Ugt2b35, Sult5a1, Gstm3, and Mrp4), CAR (Cyp2b10, Aldh1a1, Aldh1a7, Ugt1a1, Ugt2b34, Sult1e1, Sult3a1, Sult5a1, Papps2, Gstt1, Gsta1, Gsta4, Gstm1-4, and Mrp2-4), PXR (Cyp3a11, Ugt1a1, Ugt1a5, Ugt1a9, Gsta1, Gstm1-m3, Oatp1a4, and Mrp3), PPARα (Cyp4a14, Aldh1a1, mGst3, Gstm4, and Mrp4), and Nrf2 (Nqo1, Aldh1a1, Gsta1, Gsta4, Gstm1-m4, mGst3, and Mrp3-4). Taken together, these data reveal transcription factor specificity and overlap in regulating hepatic drug disposition genes by chemical activators. Coordinated regulation of phase I, phase II, and transport genes by activators of transcription factors can have implications in development of pharmaceuticals as well as risk assessment of environmental contaminants.
Figures


References
-
- Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, Gonzalez FJ, Peters JM. (2001) Peroxisome proliferator-activated receptor-α regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J Biol Chem 276:39088–39093 - PubMed
-
- Aleksunes LM, Manautou JE. (2007) Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol 35:459–473 - PubMed
-
- Alnouti Y, Klaassen CD. (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93:242–255 - PubMed
-
- Alnouti Y, Klaassen CD. (2008a) Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther 324:612–621 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR021940/RR/NCRR NIH HHS/United States
- P30-ES005022/ES/NIEHS NIH HHS/United States
- T32 ES007079/ES/NIEHS NIH HHS/United States
- R01 DK081461/DK/NIDDK NIH HHS/United States
- ES020522/ES/NIEHS NIH HHS/United States
- R01 ES009649/ES/NIEHS NIH HHS/United States
- P20 RR021940/RR/NCRR NIH HHS/United States
- R01 ES019487/ES/NIEHS NIH HHS/United States
- DK081461/DK/NIDDK NIH HHS/United States
- K99 DK080774/DK/NIDDK NIH HHS/United States
- ES007079/ES/NIEHS NIH HHS/United States
- ES009649/ES/NIEHS NIH HHS/United States
- DK080774/DK/NIDDK NIH HHS/United States
- R01 ES020522/ES/NIEHS NIH HHS/United States
- R00 DK080774/DK/NIDDK NIH HHS/United States
- ES019487/ES/NIEHS NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

