ENaC inhibition stimulates Cl- secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism
- PMID: 22496413
- PMCID: PMC3431594
- DOI: 10.1152/ajprenal.00030.2012
ENaC inhibition stimulates Cl- secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism
Abstract
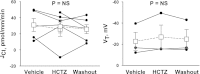
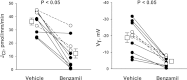
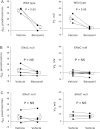
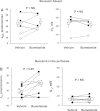
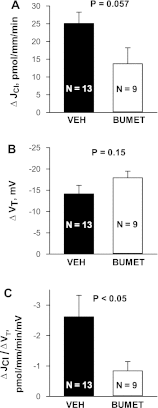
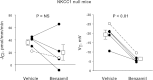
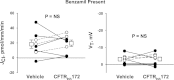
In cortical collecting ducts (CCDs) perfused in vitro, inhibiting the epithelial Na(+) channel (ENaC) reduces Cl(-) absorption. Since ENaC does not transport Cl(-), the purpose of this study was to determine how ENaC modulates Cl(-) absorption. Thus, Cl(-) absorption was measured in CCDs perfused in vitro that were taken from mice given aldosterone for 7 days. In wild-type mice, we observed no effect of luminal hydrochlorothiazide on either Cl(-) absorption or transepithelial voltage (V(T)). However, application of an ENaC inhibitor [benzamil (3 μM)] to the luminal fluid or application of a Na(+)-K(+)-ATPase inhibitor to the bath reduced Cl(-) absorption by ∼66-75% and nearly obliterated lumen-negative V(T). In contrast, ENaC inhibition had no effect in CCDs from collecting duct-specific ENaC-null mice (Hoxb7:CRE, Scnn1a(loxlox)). Whereas benzamil-sensitive Cl(-) absorption did not depend on CFTR, application of a Na(+)-K(+)-2Cl(-) cotransport inhibitor (bumetanide) to the bath or ablation of the gene encoding Na(+)-K(+)-2Cl(-) cotransporter 1 (NKCC1) blunted benzamil-sensitive Cl(-) absorption, although the benzamil-sensitive component of V(T) was unaffected. In conclusion, first, in CCDs from aldosterone-treated mice, most Cl(-) absorption is benzamil sensitive, whereas thiazide-sensitive Cl(-) absorption is undetectable. Second, benzamil-sensitive Cl(-) absorption occurs by inhibition of ENaC, possibly due to elimination of lumen-negative V(T). Finally, benzamil-sensitive Cl(-) flux occurs, at least in part, through transcellular transport through a pathway that depends on NKCC1.
Figures







Similar articles
-
ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. I. Stilbene-sensitive Cl- secretion.Am J Physiol Renal Physiol. 2015 Aug 1;309(3):F251-8. doi: 10.1152/ajprenal.00471.2013. Epub 2015 Apr 29. Am J Physiol Renal Physiol. 2015. PMID: 25925258 Free PMC article.
-
ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. II. Bafilomycin-sensitive H+ secretion.Am J Physiol Renal Physiol. 2015 Aug 1;309(3):F259-68. doi: 10.1152/ajprenal.00120.2015. Epub 2015 May 27. Am J Physiol Renal Physiol. 2015. PMID: 26017972 Free PMC article.
-
Nitric oxide reduces Cl⁻ absorption in the mouse cortical collecting duct through an ENaC-dependent mechanism.Am J Physiol Renal Physiol. 2013 Jun 1;304(11):F1390-7. doi: 10.1152/ajprenal.00292.2012. Epub 2013 Mar 20. Am J Physiol Renal Physiol. 2013. PMID: 23515718 Free PMC article.
-
Cortical distal nephron Cl(-) transport in volume homeostasis and blood pressure regulation.Am J Physiol Renal Physiol. 2013 Aug 15;305(4):F427-38. doi: 10.1152/ajprenal.00022.2013. Epub 2013 May 1. Am J Physiol Renal Physiol. 2013. PMID: 23637202 Free PMC article. Review.
-
[Regulation of kidney on potassium balance and its clinical significance].Sheng Li Xue Bao. 2023 Apr 25;75(2):216-230. Sheng Li Xue Bao. 2023. PMID: 37089096 Review. Chinese.
Cited by
-
Reduced ENaC activity and blood pressure in mice with genetic knockout of the insulin receptor in the renal collecting duct.Am J Physiol Renal Physiol. 2013 Feb 1;304(3):F279-88. doi: 10.1152/ajprenal.00161.2012. Epub 2012 Nov 28. Am J Physiol Renal Physiol. 2013. PMID: 23195676 Free PMC article.
-
NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct.Am J Physiol Renal Physiol. 2015 Feb 1;308(3):F244-51. doi: 10.1152/ajprenal.00596.2013. Epub 2014 Nov 12. Am J Physiol Renal Physiol. 2015. PMID: 25391901 Free PMC article.
-
Renal intercalated cells and blood pressure regulation.Kidney Res Clin Pract. 2017 Dec;36(4):305-317. doi: 10.23876/j.krcp.2017.36.4.305. Epub 2017 Dec 31. Kidney Res Clin Pract. 2017. PMID: 29285423 Free PMC article. Review.
-
Pendrin localizes to the adrenal medulla and modulates catecholamine release.Am J Physiol Endocrinol Metab. 2015 Sep 15;309(6):E534-45. doi: 10.1152/ajpendo.00035.2015. Epub 2015 Jul 14. Am J Physiol Endocrinol Metab. 2015. PMID: 26173457 Free PMC article.
-
The Role of Intercalated Cell Nedd4-2 in BP Regulation, Ion Transport, and Transporter Expression.J Am Soc Nephrol. 2018 Jun;29(6):1706-1719. doi: 10.1681/ASN.2017080826. Epub 2018 May 17. J Am Soc Nephrol. 2018. PMID: 29773687 Free PMC article.
References
-
- Chalfant ML, Peterson-Yantorno K, O'Brien TG, Civan MM. Regulation of epithelial Na+ channels from M-1 cortical collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F861–F870, 1996 - PubMed
-
- Eladari D, Teulon J. A new regulator of the vacuolar H+-ATPase in the kidney. Kidney Int 80: 907–909, 2011 - PubMed
-
- Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+-K+-2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

