Lamin B1 loss is a senescence-associated biomarker
- PMID: 22496421
- PMCID: PMC3364172
- DOI: 10.1091/mbc.E11-10-0884
Lamin B1 loss is a senescence-associated biomarker
Abstract
Cellular senescence is a potent tumor-suppressive mechanism that arrests cell proliferation and has been linked to aging. However, studies of senescence have been impeded by the lack of simple, exclusive biomarkers of the senescent state. Senescent cells develop characteristic morphological changes, which include enlarged and often irregular nuclei and chromatin reorganization. Because alterations to the nuclear lamina can affect both nuclear morphology and gene expression, we examined the nuclear lamina of senescent cells. We show here than lamin B1 is lost from primary human and murine cell strains when they are induced to senesce by DNA damage, replicative exhaustion, or oncogene expression. Lamin B1 loss did not depend on the p38 mitogen-activated protein kinase, nuclear factor-κB, ataxia telangiectasia-mutated kinase, or reactive oxygen species signaling pathways, which are positive regulators of senescent phenotypes. However, activation of either the p53 or pRB tumor suppressor pathway was sufficient to induce lamin B1 loss. Lamin B1 declined at the mRNA level via a decrease in mRNA stability rather than by the caspase-mediated degradation seen during apoptosis. Last, lamin B1 protein and mRNA declined in mouse tissue after senescence was induced by irradiation. Our findings suggest that lamin B1 loss can serve as biomarker of senescence both in culture and in vivo.
Figures


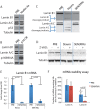
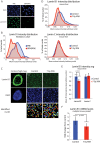
Similar articles
-
p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes.J Cell Biol. 2013 May 13;201(4):613-29. doi: 10.1083/jcb.201206006. Epub 2013 May 6. J Cell Biol. 2013. PMID: 23649808 Free PMC article.
-
Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation.EMBO J. 2012 Mar 7;31(5):1080-94. doi: 10.1038/emboj.2011.492. Epub 2012 Jan 13. EMBO J. 2012. PMID: 22246186 Free PMC article.
-
Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence.Nucleus. 2012 Sep-Oct;3(5):411-7. doi: 10.4161/nucl.21674. Epub 2012 Aug 16. Nucleus. 2012. PMID: 22895091 Free PMC article.
-
Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP).Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182507 Review.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
-
Human RTEL1 Interacts with KPNB1 (Importin β) and NUP153 and Connects Nuclear Import to Nuclear Envelope Stability in S-Phase.Cells. 2023 Dec 8;12(24):2798. doi: 10.3390/cells12242798. Cells. 2023. PMID: 38132118 Free PMC article.
-
Gene-rich chromosomal regions are preferentially localized in the lamin B deficient nuclear blebs of atypical progeria cells.Nucleus. 2015;6(1):66-76. doi: 10.1080/19491034.2015.1004256. Nucleus. 2015. PMID: 25738644 Free PMC article.
-
Comparative biological activity of palbociclib and ribociclib in hormone receptor-positive breast cancer.Sci Rep. 2024 Jul 11;14(1):16030. doi: 10.1038/s41598-024-67126-2. Sci Rep. 2024. PMID: 38992220 Free PMC article.
-
Impaired neuron differentiation in GBA-associated Parkinson's disease is linked to cell cycle defects in organoids.NPJ Parkinsons Dis. 2023 Dec 18;9(1):166. doi: 10.1038/s41531-023-00616-8. NPJ Parkinsons Dis. 2023. PMID: 38110400 Free PMC article.
-
Upregulation of YPEL3 expression and induction of human breast cancer cell death by microRNAs.Toxicol Res. 2024 Jun 21;40(4):599-611. doi: 10.1007/s43188-024-00251-2. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345743 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

