Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner
- PMID: 22496425
- PMCID: PMC3364179
- DOI: 10.1091/mbc.E11-10-0844
Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner
Abstract
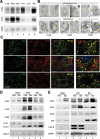
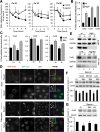
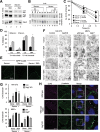
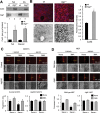
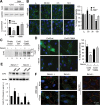
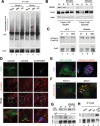
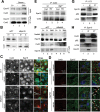
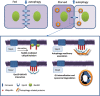
Different pathways contribute to the turnover of connexins, the main structural components of gap junctions (GJs). The cellular pool of connexins targeted to each pathway and the functional consequences of degradation through these degradative pathways are unknown. In this work, we focused on the contribution of macroautophagy to connexin degradation. Using pharmacological and genetic blockage of macroautophagy both in vitro and in vivo, we found that the cellular pool targeted by this autophagic system is primarily the one organized into GJs. Interruption of connexins' macroautophagy resulted in their retention at the plasma membrane in the form of functional GJs and subsequent increased GJ-mediated intercellular diffusion. Up-regulation of macroautophagy alone is not sufficient to induce connexin internalization and degradation. To better understand what factors determine the autophagic degradation of GJ connexins, we analyzed the changes undergone by the fraction of plasma membrane connexin 43 targeted for macroautophagy and the sequence of events that trigger this process. We found that Nedd4-mediated ubiquitinylation of the connexin molecule is required to recruit the adaptor protein Eps15 to the GJ and to initiate the autophagy-dependent internalization and degradation of connexin 43. This study reveals a novel regulatory role for macroautophagy in GJ function that is directly dependent on the ubiquitinylation of plasma membrane connexins.
Figures
References
-
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62:256–267. - PubMed
-
- Bugnicourt A, Mari M, Reggiori F, Haguenauer-Tsapis R, Galan JM. Irs4p and Tax4p: two redundant EH domain proteins involved in autophagy. Traffic. 2008;9:755–769. - PubMed
-
- Catarino S, Ramalho JS, Marques C, Pereira P, Girao H. Ubiquitin-mediated internalization of connexin43 is independent of the canonical endocytic tyrosine-sorting signal. Biochem J. 2011;437:255–267. - PubMed
-
- Csikos G, Lippai M, Lukacsovich T, Juhasz G, Henn L, Erdelyi M, Maroy P, Sass M. A novel role for the Drosophila epsin (lqf): involvement in autophagy. Autophagy. 2009;5:636–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

