NaJAZh regulates a subset of defense responses against herbivores and spontaneous leaf necrosis in Nicotiana attenuata plants
- PMID: 22496510
- PMCID: PMC3375940
- DOI: 10.1104/pp.112.193771
NaJAZh regulates a subset of defense responses against herbivores and spontaneous leaf necrosis in Nicotiana attenuata plants
Abstract
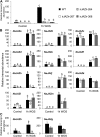
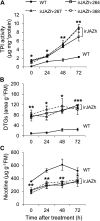
The JASMONATE ZIM DOMAIN (JAZ) proteins function as negative regulators of jasmonic acid signaling in plants. We cloned 12 JAZ genes from native tobacco (Nicotiana attenuata), including nine novel JAZs in tobacco, and examined their expression in plants that had leaves elicited by wounding or simulated herbivory. Most JAZ genes showed strong expression in the elicited leaves, but NaJAZg was mainly expressed in roots. Another novel herbivory-elicited gene, NaJAZh, was analyzed in detail. RNA interference suppression of this gene in inverted-repeat (ir)JAZh plants deregulated a specific branch of jasmonic acid-dependent direct and indirect defenses: irJAZh plants showed greater trypsin protease inhibitor activity, 17-hydroxygeranyllinalool diterpene glycosides accumulation, and emission of volatile organic compounds from leaves. Silencing of NaJAZh also revealed a novel cross talk in JAZ-regulated secondary metabolism, as irJAZh plants had significantly reduced nicotine levels. In addition, irJAZh spontaneously developed leaf necrosis during the transition to flowering. Because the lesions closely correlated with the elevated expression of programmed cell death genes and the accumulations of salicylic acid and hydrogen peroxide in the leaves, we propose a novel role of the NaJAZh protein as a repressor of necrosis and/or programmed cell death during plant development.
Figures










References
-
- Allmann S, Baldwin IT. (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329: 1075–1078 - PubMed
-
- Balbi V, Devoto A. (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177: 301–318 - PubMed
-
- Baldwin IT, Kessler A, Halitschke R. (2002) Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol 5: 351–354 - PubMed
-
- Baldwin IT, Zhang ZP, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. (1997) Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201: 397–404
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

