The function and molecular identity of inward rectifier channels in vestibular hair cells of the mouse inner ear
- PMID: 22496522
- PMCID: PMC3434605
- DOI: 10.1152/jn.00098.2012
The function and molecular identity of inward rectifier channels in vestibular hair cells of the mouse inner ear
Abstract
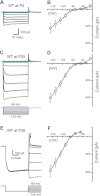
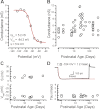
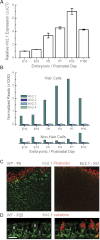
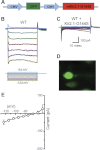
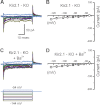
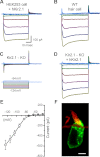
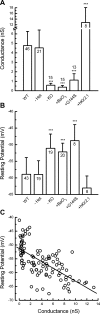
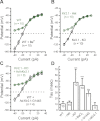
Inner ear hair cells respond to mechanical stimuli with graded receptor potentials. These graded responses are modulated by a host of voltage-dependent currents that flow across the basolateral membrane. Here, we examine the molecular identity and the function of a class of voltage-dependent ion channels that carries the potassium-selective inward rectifier current known as I(K1). I(K1) has been identified in vestibular hair cells of various species, but its molecular composition and functional contributions remain obscure. We used quantitative RT-PCR to show that the inward rectifier gene, Kir2.1, is highly expressed in mouse utricle between embryonic day 15 and adulthood. We confirmed Kir2.1 protein expression in hair cells by immunolocalization. To examine the molecular composition of I(K1), we recorded voltage-dependent currents from type II hair cells in response to 50-ms steps from -124 to -54 in 10-mV increments. Wild-type cells had rapidly activating inward currents with reversal potentials close to the K(+) equilibrium potential and a whole-cell conductance of 4.8 ± 1.5 nS (n = 46). In utricle hair cells from Kir2.1-deficient (Kir2.1(-/-)) mice, I(K1) was absent at all stages examined. To identify the functional contribution of Kir2.1, we recorded membrane responses in current-clamp mode. Hair cells from Kir2.1(-/-) mice had significantly (P < 0.001) more depolarized resting potentials and larger, slower membrane responses than those of wild-type cells. These data suggest that Kir2.1 is required for I(K1) in type II utricle hair cells and contributes to hyperpolarized resting potentials and fast, small amplitude receptor potentials in response to current inputs, such as those evoked by hair bundle deflections.
Figures


Similar articles
-
Molecular characterization of an inward rectifier channel (IKir) found in avian vestibular hair cells: cloning and expression of pKir2.1.Physiol Genomics. 2004 Oct 4;19(2):155-69. doi: 10.1152/physiolgenomics.00096.2004. Epub 2004 Aug 17. Physiol Genomics. 2004. PMID: 15316115
-
Elementary properties of Kir2.1, a strong inwardly rectifying K(+) channel expressed by pigeon vestibular type II hair cells.Neuroscience. 2008 Sep 9;155(4):1250-61. doi: 10.1016/j.neuroscience.2008.06.048. Epub 2008 Jul 1. Neuroscience. 2008. PMID: 18652879
-
Channeling your inner ear potassium: K(+) channels in vestibular hair cells.Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review.
-
Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: evidence for a major role of Kir2.4 subunits.J Membr Biol. 2006;213(1):19-29. doi: 10.1007/s00232-006-0037-y. Epub 2007 Mar 8. J Membr Biol. 2006. PMID: 17347781 Free PMC article.
-
Cardiac strong inward rectifier potassium channels.J Mol Cell Cardiol. 2010 Jan;48(1):45-54. doi: 10.1016/j.yjmcc.2009.08.013. Epub 2009 Aug 22. J Mol Cell Cardiol. 2010. PMID: 19703462 Free PMC article. Review.
Cited by
-
Human fetal inner ear involvement in congenital cytomegalovirus infection.Acta Neuropathol Commun. 2013 Oct 2;1:63. doi: 10.1186/2051-5960-1-63. Acta Neuropathol Commun. 2013. PMID: 24252374 Free PMC article.
-
SHIELD: an integrative gene expression database for inner ear research.Database (Oxford). 2015 Jul 24;2015:bav071. doi: 10.1093/database/bav071. Print 2015. Database (Oxford). 2015. PMID: 26209310 Free PMC article.
-
Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons.J Gen Physiol. 2014 Apr;143(4):481-97. doi: 10.1085/jgp.201311126. Epub 2014 Mar 17. J Gen Physiol. 2014. PMID: 24638995 Free PMC article.
-
Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells.Nat Biotechnol. 2017 Jun;35(6):583-589. doi: 10.1038/nbt.3840. Epub 2017 May 1. Nat Biotechnol. 2017. PMID: 28459451 Free PMC article.
-
The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells.Elife. 2024 Dec 3;13:RP94342. doi: 10.7554/eLife.94342. Elife. 2024. PMID: 39625061 Free PMC article.
References
-
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol 90: 155–164, 2003 - PubMed
-
- Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K channels. Nat Rev Neurosci 4: 957–967, 2003 - PubMed
-
- Brichta AM, Aubert A, Eatock RA, Goldberg JM. Regional analysis of whole cell currents from hair cells of the turtle posterior crista. J Neurophysiol 88: 3259–3278, 2002 - PubMed
-
- Correia MJ, Wood TG, Prusak D, Weng T, Rennie KJ, Wang HQ. Molecular characterization of an inward rectifier channel (IKir) found in avian vestibular hair cells: cloning, and expression of pKir2.1. Physiol Genomics 19: 155–169, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

