Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-β revealed by a novel fluorescence resonance energy transfer assay
- PMID: 22496575
- PMCID: PMC3354533
- DOI: 10.1523/JNEUROSCI.0227-12.2012
Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-β revealed by a novel fluorescence resonance energy transfer assay
Abstract
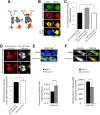
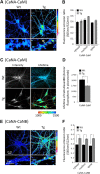
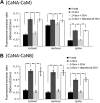
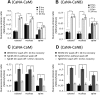
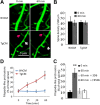
Calcineurin (CaN) activation is critically involved in the regulation of spine morphology in response to oligomeric amyloid-β (Aβ) as well as in synaptic plasticity in normal memory, but no existing techniques can monitor the spatiotemporal pattern of CaN activity. Here, we use a spectral fluorescence resonance energy transfer approach to monitor CaN activation dynamics in real time with subcellular resolution. When oligomeric Aβ derived from Tg2576 murine transgenic neurons or human AD brains were applied to wild-type murine primary cortical neurons, we observe a dynamic progression of CaN activation within minutes, first in dendritic spines, and then in the cytoplasm and, in hours, in the nucleus. CaN activation in spines leads to rapid but reversible morphological changes in spines and in postsynaptic proteins; longer exposure leads to NFAT (nuclear factor of activated T-cells) translocation to the nucleus and frank spine loss. These results provide a framework for understanding the role of calcineurin in synaptic alterations associated with AD pathogenesis.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
