Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression
- PMID: 22496622
- PMCID: PMC3323900
- DOI: 10.1593/neo.111570
Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression
Abstract
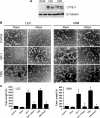
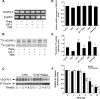
Mammalian target of rapamycin (mTOR) controls lymphangiogenesis. However, the underlying mechanism is not clear. Here we show that rapamycin suppressed insulin-like growth factor 1 (IGF-1)- or fetal bovine serum (FBS)-stimulated lymphatic endothelial cell (LEC) tube formation, an in vitro model of lymphangiogenesis. Expression of a rapamycin-resistant and kinase-active mTOR (S2035T, mTOR-T), but not a rapamycin-resistant and kinase-dead mTOR (S2035T/D2357E, mTOR-TE), conferred resistance to rapamycin inhibition of LEC tube formation, suggesting that rapamycin inhibition of LEC tube formation is mTOR kinase activity dependent. Also, rapamycin inhibited proliferation and motility in the LECs. Furthermore, we found that rapamycin inhibited protein expression of VEGF receptor 3 (VEGFR-3) by inhibiting protein synthesis and promoting protein degradation of VEGFR-3 in the cells. Down-regulation of VEGFR-3 mimicked the effect of rapamycin, inhibiting IGF-1- or FBS-stimulated tube formation, whereas over-expression of VEGFR-3 conferred high resistance to rapamycin inhibition of LEC tube formation. The results indicate that rapamycin inhibits LEC tube formation at least in part by downregulating VEGFR-3 protein expression.
Copyright © 2012 Neoplasia Press, Inc.
Figures




References
-
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. - PubMed
-
- Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem. 2010;17:698–708. - PubMed
-
- Proud CG. mTOR signalling in health and disease. Biochem Soc Trans. 2011;39:431–436. - PubMed
-
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
