Leishmania induces survival, proliferation and elevated cellular dNTP levels in human monocytes promoting acceleration of HIV co-infection
- PMID: 22496656
- PMCID: PMC3320607
- DOI: 10.1371/journal.ppat.1002635
Leishmania induces survival, proliferation and elevated cellular dNTP levels in human monocytes promoting acceleration of HIV co-infection
Abstract
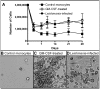
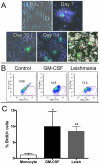
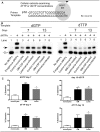
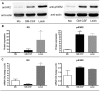
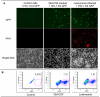
Leishmaniasis is a parasitic disease that is widely prevalent in many tropical and sub-tropical regions of the world. Infection with Leishmania has been recognized to induce a striking acceleration of Human Immunodeficiency Virus Type 1 (HIV-1) infection in coinfected individuals through as yet incompletely understood mechanisms. Cells of the monocyte/macrophage lineage are the predominant cell types coinfected by both pathogens. Monocytes and macrophages contain extremely low levels of deoxynucleoside triphosphates (dNTPs) due to their lack of cell cycling and S phase, where dNTP biosynthesis is specifically activated. Lentiviruses, such as HIV-1, are unique among retroviruses in their ability to replicate in these non-dividing cells due, at least in part, to their highly efficient reverse transcriptase (RT). Nonetheless, viral replication progresses more efficiently in the setting of higher intracellular dNTP concentrations related to enhanced enzyme kinetics of the viral RT. In the present study, in vitro infection of CD14+ peripheral blood-derived human monocytes with Leishmania major was found to induce differentiation, marked elevation of cellular p53R2 ribonucleotide reductase subunit and R2 subunit expression. The R2 subunit is restricted to the S phase of the cell cycle. Our dNTP assay demonstrated significant elevation of intracellular monocyte-derived macrophages (MDMs) dNTP concentrations in Leishmania-infected cell populations as compared to control cells. Infection of Leishmania-maturated MDMs with a pseudotyped GFP expressing HIV-1 resulted in increased numbers of GFP+ cells in the Leishmania-maturated MDMs as compared to control cells. Interestingly, a sub-population of Leishmania-maturated MDMs was found to have re-entered the cell cycle, as demonstrated by BrdU labeling. In conclusion, Leishmania infection of primary human monocytes promotes the induction of an S phase environment and elevated dNTP levels with notable elevation of HIV-1 expression in the setting of coinfection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

