Neurodegeneration in Alzheimer disease: role of amyloid precursor protein and presenilin 1 intracellular signaling
- PMID: 22496686
- PMCID: PMC3306972
- DOI: 10.1155/2012/187297
Neurodegeneration in Alzheimer disease: role of amyloid precursor protein and presenilin 1 intracellular signaling
Abstract
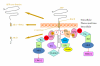
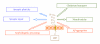
Alzheimer disease (AD) is a heterogeneous neurodegenerative disorder characterized by (1) progressive loss of synapses and neurons, (2) intracellular neurofibrillary tangles, composed of hyperphosphorylated Tau protein, and (3) amyloid plaques. Genetically, AD is linked to mutations in few proteins amyloid precursor protein (APP) and presenilin 1 and 2 (PS1 and PS2). The molecular mechanisms underlying neurodegeneration in AD as well as the physiological function of APP are not yet known. A recent theory has proposed that APP and PS1 modulate intracellular signals to induce cell-cycle abnormalities responsible for neuronal death and possibly amyloid deposition. This hypothesis is supported by the presence of a complex network of proteins, clearly involved in the regulation of signal transduction mechanisms that interact with both APP and PS1. In this review we discuss the significance of novel finding related to cell-signaling events modulated by APP and PS1 in the development of neurodegeneration.
Figures

References
-
- Golde TE, Eckman CB. Physiologic and pathologic events mediated by intramembranous and juxtamembranous proteolysis. Science’s STKE. 2003;2003(172):p. RE4. - PubMed
-
- Selkoe DJ, Podlisny MB. Deciphering the genetic basis of Alzheimer’s disease. Annual Review of Genomics and Human Genetics. 2002;3:67–99. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. - PubMed
-
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131(2):215–221. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

