Differential inflammatory response to inhaled lipopolysaccharide targeted either to the airways or the alveoli in man
- PMID: 22496751
- PMCID: PMC3319549
- DOI: 10.1371/journal.pone.0033505
Differential inflammatory response to inhaled lipopolysaccharide targeted either to the airways or the alveoli in man
Abstract
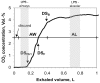
Endotoxin (Lipopolysaccharide, LPS) is a potent inducer of inflammation and there is various LPS contamination in the environment, being a trigger of lung diseases and exacerbation. The objective of this study was to assess the time course of inflammation and the sensitivities of the airways and alveoli to targeted LPS inhalation in order to understand the role of LPS challenge in airway disease.In healthy volunteers without any bronchial hyperresponsiveness we targeted sequentially 1, 5 and 20 µg LPS to the airways and 5 µg LPS to the alveoli using controlled aerosol bolus inhalation. Inflammatory parameters were assessed during a 72 h time period. LPS deposited in the airways induced dose dependent systemic responses with increases of blood neutrophils (peaking at 6 h), Interleukin-6 (peaking at 6 h), body temperature (peaking at 12 h), and CRP (peaking at 24 h). 5 µg LPS targeted to the alveoli caused significantly stronger effects compared to 5 µg airway LPS deposition. Local responses were studied by measuring lung function (FEV(1)) and reactive oxygen production, assessed by hydrogen peroxide (H(2)O(2)) in fractionated exhaled breath condensate (EBC). FEV(1) showed a dose dependent decline, with lowest values at 12 h post LPS challenge. There was a significant 2-fold H(2)O(2) induction in airway-EBC at 2 h post LPS inhalation. Alveolar LPS targeting resulted in the induction of very low levels of EBC-H(2)O(2).Targeting LPS to the alveoli leads to stronger systemic responses compared to airway LPS targeting. Targeted LPS inhalation may provide a novel model of airway inflammation for studying the role of LPS contamination of air pollution in lung diseases, exacerbation and anti-inflammatory drugs.
Conflict of interest statement
Figures

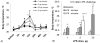
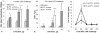


References
-
- Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. - PubMed
-
- Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. - PubMed
-
- Maris NA, Dessing MC, de Vos AF, Bresser P, van der Zee JS, et al. Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur Respir J. 2006;28:622–626. - PubMed
-
- Rietschel ET, Zahringer U, Seydel U, Ulmer AJ. Bacterial endotoxins: Bioactive conformation and specific host-recognition. Shock. 1999;12:21–21.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

