Epidermal transglutaminase (TGase 3) is required for proper hair development, but not the formation of the epidermal barrier
- PMID: 22496784
- PMCID: PMC3319564
- DOI: 10.1371/journal.pone.0034252
Epidermal transglutaminase (TGase 3) is required for proper hair development, but not the formation of the epidermal barrier
Abstract
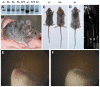
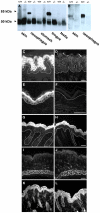
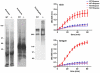
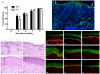
Transglutaminases (TGase), a family of cross-linking enzymes present in most cell types, are important in events as diverse as cell-signaling and matrix stabilization. Transglutaminase 1 is crucial in developing the epidermal barrier, however the skin also contains other family members, in particular TGase 3. This isoform is highly expressed in the cornified layer, where it is believed to stabilize the epidermis and its reduction is implicated in psoriasis. To understand the importance of TGase 3 in vivo we have generated and analyzed mice lacking this protein. Surprisingly, these animals display no obvious defect in skin development, no overt changes in barrier function or ability to heal wounds. In contrast, hair lacking TGase 3 is thinner, has major alterations in the cuticle cells and hair protein cross-linking is markedly decreased. Apparently, while TGase 3 is of unique functional importance in hair, in the epidermis loss of TGase 3 can be compensated for by other family members.
Conflict of interest statement
Figures





References
-
- Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000;41(1):1–27. - PubMed
-
- Aeschlimann D, Paulsson M. Transglutaminases: protein cross-linking enzymes in tissues and body fluids. Thromb Haemost. 1994;71(4):402–15. - PubMed
-
- Mycek MJ, Clarke DD, Neidle A, Waelsch H. Amine incorporation into insulin as catalyzed by transglutaminase. Arch Biochem Biophys. 1959;84:528–40. - PubMed
-
- Folk JE, Cole PW. Structural Requirements Of Specific Substrates For Guinea Pig Liver Transglutaminase. J Biol Chem. 1965;240:2951–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

