A family-wide RT-PCR assay for detection of paramyxoviruses and application to a large-scale surveillance study
- PMID: 22496880
- PMCID: PMC3319594
- DOI: 10.1371/journal.pone.0034961
A family-wide RT-PCR assay for detection of paramyxoviruses and application to a large-scale surveillance study
Abstract
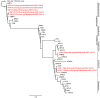
Family-wide molecular diagnostic assays are valuable tools for initial identification of viruses during outbreaks and to limit costs of surveillance studies. Recent discoveries of paramyxoviruses have called for such assay that is able to detect all known and unknown paramyxoviruses in one round of PCR amplification. We have developed a RT-PCR assay consisting of a single degenerate primer set, able to detect all members of the Paramyxoviridae family including all virus genera within the subfamilies Paramyxovirinae and Pneumovirinae. Primers anneal to domain III of the polymerase gene, with the 3' end of the reverse primer annealing to the conserved motif GDNQ, which is proposed to be the active site for nucleotide polymerization. The assay was fully optimized and was shown to indeed detect all available paramyxoviruses tested. Clinical specimens from hospitalized patients that tested positive for known paramyxoviruses in conventional assays were also detected with the novel family-wide test. A high-throughput fluorescence-based RT-PCR version of the assay was developed for screening large numbers of specimens. A large number of samples collected from wild birds was tested, resulting in the detection of avian paramyxoviruses type 1 in both barnacle and white-fronted geese, and type 8 in barnacle geese. Avian metapneumovirus type C was found for the first time in Europe in mallards, greylag geese and common gulls. The single round family-wide RT-PCR assay described here is a useful tool for the detection of known and unknown paramyxoviruses, and screening of large sample collections from humans and animals.
Conflict of interest statement
Figures



References
-
- Lamb RA, Parks GD. Paramyxoviridae. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 1449–1496.
-
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. - PubMed
-
- Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. - PubMed
-
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

