Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion
- PMID: 22496888
- PMCID: PMC3319627
- DOI: 10.1371/journal.pone.0035036
Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion
Abstract
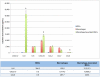
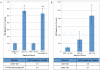
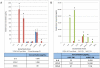
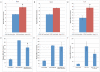
Mesenchymal stem cells (MSCs) exhibit tropism for sites of tissue injury and tumors. However, the influence of the microenvironment on MSC phenotype and localization remains incompletely characterized. In this study, we begin to define a macrophage-induced MSC phenotype. These MSCs secrete interleukin-6 (IL-6), CCL5, and interferon gamma-induced protein-10 (CXCL10) and exhibit increased mobility in response to multiple soluble factors produced by macrophages including IL-8, CCL2, and CCL5. The pro-migratory phenotype is dependent on activation of a c-Jun N-terminal kinase (JNK) pathway. This work begins to identify the influence of macrophages on MSC biology. These interactions are likely to play an important role in the tissue inflammatory response and may provide insight into the migratory potential of MSCs in inflammation and tissue injury.
Conflict of interest statement
Figures


References
-
- Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. - PubMed
-
- Ryu CH, Park SH, Park SA, Kim SM, Lim JY, et al. Gene Therapy of Intracranial Glioma Using Interleukin 12-Secreting Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Human gene therapy, 22(6) 2011;733–43 - PubMed
-
- Bak XY, Dang HL, Yang J, Ye K, Lee EX, et al. Human Embryonic Stem Cell-derived Mesenchymal Stem Cells as Cellular Delivery Vehicles for Prodrug Gene Therapy of Glioblastoma. Human gene therapy. 2011;22(11):1365–77. - PubMed
-
- Yang B, Wu X, Mao Y, Bao W, Gao L, et al. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. - PubMed
-
- Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005;15:417–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

