Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells
- PMID: 22496903
- PMCID: PMC3322149
- DOI: 10.1371/journal.pone.0035155
Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells
Erratum in
- PLoS One. 2012;7(5): doi/10.1371/annotation/12a8fc89-5f47-4bad-8863-863d99a0e52d
Abstract
Background: Compound A (CpdA) is a dissociating non-steroidal glucocorticoid receptor (GR) ligand which has anti-inflammatory properties exerted by down-modulating proinflammatory gene expression. By favouring GR monomer formation, CpdA does not enhance glucocorticoid (GC) response element-driven gene expression, resulting in a reduced side effect profile as compared to GCs. Considering the importance of Th1/Th2 balance in the final outcome of immune and inflammatory responses, we analyzed how selective GR modulation differentially regulates the activity of T-bet and GATA-3, master drivers of Th1 and Th2 differentiation, respectively.
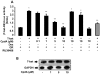
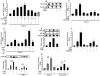
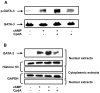
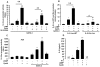
Results: Using Western analysis and reporter gene assays, we show in murine T cells that, similar to GCs, CpdA inhibits T-bet activity via a transrepressive mechanism. Different from GCs, CpdA induces GATA-3 activity by p38 MAPK-induction of GATA-3 phosphorylation and nuclear translocation. CpdA effects are reversed by the GR antagonist RU38486, proving the involvement of GR in these actions. ELISA assays demonstrate that modulation of T-bet and GATA-3 impacts on cytokine production shown by a decrease in IFN-γ and an increase in IL-5 production, respectively.
Conclusions: Taken together, through their effect favoring Th2 over Th1 responses, particular dissociated GR ligands, for which CpdA represents a paradigm, hold potential for the application in Th1-mediated immune disorders.
Conflict of interest statement
Figures


References
-
- Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007;275:98–108. - PubMed
-
- De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10:497–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

