Cyclodextrin-containing polymers: versatile platforms of drug delivery materials
- PMID: 22496980
- PMCID: PMC3307009
- DOI: 10.1155/2012/262731
Cyclodextrin-containing polymers: versatile platforms of drug delivery materials
Abstract
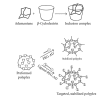
Nanoparticles are being widely explored as potential therapeutics for numerous applications in medicine and have been shown to significantly improve the circulation, biodistribution, efficacy, and safety profiles of multiple classes of drugs. One leading class of nanoparticles involves the use of linear, cyclodextrin-containing polymers (CDPs). As is discussed in this paper, CDPs can incorporate therapeutic payloads into nanoparticles via covalent attachment of prodrug/drug molecules to the polymer (the basis of the Cyclosert platform) or by noncovalent inclusion of cationic CDPs to anionic, nucleic acid payloads (the basis of the RONDEL platform). For each of these two approaches, we review the relevant molecular architecture and its rationale, discuss the physicochemical and biological properties of these nanoparticles, and detail the progress of leading drug candidates for each that have achieved clinical evaluation. Finally, we look ahead to potential future directions of investigation and product candidates based upon this technology.
Figures





References
-
- Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nature Reviews Drug Discovery. 2004;3(12):1023–1035. - PubMed
-
- Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discovery Today. 2005;10(21):1451–1458. - PubMed
-
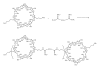
- Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Synthesis of linear, β-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjugate Chemistry. 2003;14(5):1007–1017. - PubMed
-
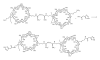
- Cheng J, Khin KT, Davis ME. Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates. Molecular Pharmacology. 2004;1(3):183–193. - PubMed
-
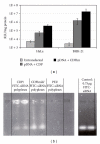
- Schluep T, Gunawan P, Ma L, et al. Polymeric tubulysin-peptide nanoparticles with potent antitumor activity. Clinical Cancer Research. 2009;15(1):181–189. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

