Malformations of cortical development
- PMID: 22497611
- PMCID: PMC8092961
- DOI: 10.1111/j.1750-3639.2012.00581.x
Malformations of cortical development
Abstract
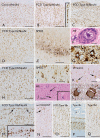
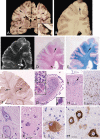
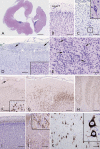
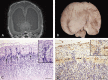
Structural abnormalities of the brain are increasingly recognized in patients that suffer from pharmacoresistant focal epilepsies by applying high-resolution imaging techniques. In many of these patients, epilepsy surgery results in control of seizures. Neuropathologically, a broad spectrum of malformations of cortical development (MCD) is observed in respective surgical brain samples. These samples provide a unique basis to further understand underlying pathomechanisms by molecular approaches and develop improved diagnostics and entirely new therapeutic perspectives. Here we provide a comprehensive description of neuropathological findings, available classification systems as well as molecular mechanisms of MCDs. We emphasize the recently published ILEA classification system for focal cortical dysplasias (FCDs), which are now histopathologically distinguished as types I to III. However, this revised classification system represents a major challenge for molecular neuropathologists, as the underlying pathomechanisms in virtually all FCD entities will need to be specified in detail. The fact that only recently, the mammalian target of rapamycin (mTOR)-antagonist Everolimus has been introduced as a treatment of epilepsies in the context of tuberous sclerosis-associated brain lesions is a striking example of a successful translational "bedside to bench and back" approach. Hopefully, the exciting clinico-pathological developments in the field of MCDs will in short term foster further therapeutic breakthroughs for the frequently associated medically refractory epilepsies.
© 2012 The Authors; Brain Pathology © 2012 International Society of Neuropathology.
Figures

References
-
- Adriaensen ME, Schaefer‐Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M (2009) Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol 16:691–696. - PubMed
-
- Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A et al (2005) The role of periventricular nodular heterotopia in epileptogenesis. Brain 128:641–651. - PubMed
-
- Aida N (1998) Fukuyama congenital muscular dystrophy: a neuroradiologic review. J Magn Reson Imaging 8:317–326. - PubMed
-
- Al‐Saleem T, Wessner LL, Scheithauer BW, Patterson K, Roach ES, Dreyer SJ et al (1998) Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83:2208–2216. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

