Axon degeneration: where the Wlds things are
- PMID: 22497934
- PMCID: PMC4449840
- DOI: 10.1016/j.cub.2012.02.056
Axon degeneration: where the Wlds things are
Abstract
Expression of the Wld(s) protein significantly delays axon degeneration in injuries and diseases, but the mechanism for this protection is unknown. Two recent reports present evidence that axonal mitochondria are required for Wld(S)-mediated axon protection.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
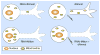
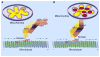
Comment on
-
A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity.Curr Biol. 2012 Apr 10;22(7):590-5. doi: 10.1016/j.cub.2012.01.065. Epub 2012 Mar 15. Curr Biol. 2012. PMID: 22425156 Free PMC article.
-
WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering.Curr Biol. 2012 Apr 10;22(7):596-600. doi: 10.1016/j.cub.2012.02.043. Epub 2012 Mar 15. Curr Biol. 2012. PMID: 22425157 Free PMC article.
References
-
- Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165:63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

