Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3
- PMID: 22498490
- PMCID: PMC3371762
- DOI: 10.18632/aging.100450
Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3
Abstract
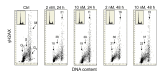
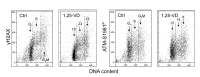
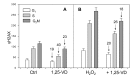
In addition to its traditional role in the regulation of calcium homeostasis and bone metabolism, vitamin D also exhibits immunomodulatory, anti-proliferative and cancer preventive activities. Molecular mechanisms that confer the chemo-preventive properties to vitamin D are poorly understood. We previously reported that constitutive phosphorylation of histone H2AX on Ser139 (γH2AX) and activation of ATM (Ser1981 phosphorylation), seen in untreated normal or tumor cells predominantly in S phase of the cell cycle, is to a large extent indicative of DNA replication stress occurring as a result of persistent DNA damage caused by endogenous oxidants, by-products of oxidative metabolism. In the present study we observed that exposure of mitogenically stimulated human lymphocytes, pulmonary carcinoma A549 and lymphoblastoid TK6 cells to 1,25-dihydroxyvitamin D3 (1,25-VD) reduced the level of constitutive expression of γH2AX and ATM-S1981P. We also observed that the H2O2-induced rise in the level of γH2AX in lymphocytes was attenuated by 1,25-VD. Whereas in lymphocytes 1,25-VD reduced by 50-70% the level of endogenous oxidants as determined by their ability to oxidize 2,7-dichlorodihydrofluorescein (DCFH) in A549 and TK6 cells the attenuation of DNA damage signaling by 1,25-VD was seen in the absence of detectable reduction in DCFH oxidation. These findings suggest that while the anti-oxidant activity of 1,25-VD may contribute to a reduction in the intensity of DNA replication stress in lymphocytes, other factors play a role in the 1,25-VD effects seen in A549 and TK6 cells. The data are consistent with the recent report on the interaction between DNA damage signaling (ATM activation) and 1,25D receptor (VDR) phosphorylation that lead to enhancement of DNA repair efficiency, and provide further support for the chemo-preventive and anti-aging properties of this vitamin/hormone.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures

References
-
- Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

