Genetic, molecular and cellular mechanisms underlying the J wave syndromes
- PMID: 22498570
- PMCID: PMC3521574
- DOI: 10.1253/circj.cj-12-0284
Genetic, molecular and cellular mechanisms underlying the J wave syndromes
Abstract
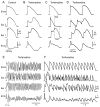
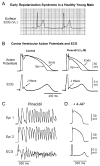
An early repolarization (ER) pattern in the ECG, distinguished by J-point elevation, slurring of the terminal part of the QRS and ST-segment elevation has long been recognized and considered to be a benign electrocardiographic manifestation. Experimental studies conducted over a decade ago suggested that some cases of ER may be associated with malignant arrhythmias. Validation of this hypothesis was provided by recent studies demonstrating that an ER pattern in the inferior or inferolateral leads is associated with increased risk for life-threatening arrhythmias, termed ER syndrome (ERS). Because accentuated J waves characterize both Brugada syndrome (BS) and ERS, these syndromes have been grouped under the term "J wave syndromes". ERS and BS share similar ECG characteristics, clinical outcomes and risk factors, as well as a common arrhythmic platform related to amplification of I(to)-mediated J waves. Although BS and ERS differ with respect to the magnitude and lead location of abnormal J wave manifestation, they can be considered to represent a continuous spectrum of phenotypic expression. Although most subjects exhibiting an ER pattern are at minimal to no risk, mounting evidence suggests that careful attention should be paid to subjects with "high risk" ER. The challenge ahead is to be able to identify those at risk for sudden cardiac death. Here I review the clinical and genetic aspects as well as the cellular and molecular mechanisms underlying the J wave syndromes.
Conflict of interest statement
Conflicts of Interest: There are no conflicts of interest to disclose.
Figures



References
-
- Tomaszewski W. Changement electrocardiographiques observes chez un homme mort de froid. Arch Mal Coeur Vaiss. 1938;31:525–528. (in French)
-
- Osborn JJ. Experimental hypothermia: Respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953;175:389–398. - PubMed
-
- Clements SD, Hurst JW. Diagnostic value of ECG abnormalities observed in subjects accidentally exposed to cold. Am J Cardiol. 1972;29:729–734. - PubMed
-
- Thompson R, Rich J, Chmelik F, Nelson WL. Evolutionary changes in the electrocardiogram of severe progressive hypothermia. J Electrocardiol. 1977;10:67–70. - PubMed
-
- Eagle K. Images in clinical medicine: Osborn waves of hypothermia. N Engl J Med. 1994;10:680. - PubMed

