High-valent organometallic copper and palladium in catalysis
- PMID: 22498623
- PMCID: PMC4384170
- DOI: 10.1038/nature11008
High-valent organometallic copper and palladium in catalysis
Abstract
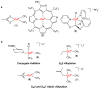
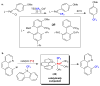
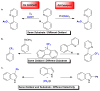
Copper and palladium catalysts are critically important in numerous commercial chemical processes. Improvements in the activity, selectivity and scope of these catalysts could drastically reduce the environmental impact, and increase the sustainability, of chemical reactions. One rapidly developing strategy for achieving these goals is to use 'high-valent' organometallic copper and palladium intermediates in catalysis. Here we describe recent advances involving both the fundamental chemistry and the applications of these high-valent metal complexes in numerous synthetically useful catalytic transformations.
Figures





References
-
- Magano J, Dunetz JR. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem Rev. 2011;111:2177–2250. - PubMed
-
- Evano G, Blanchard N, Toumi M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem Rev. 2008;108:3054–3131. - PubMed
-
- Krause N, editor. Modern Organocopper Chemistry. Wiley-VCH; Weinheim: 2002.
-
- Eckert M, Fleischmann G, Jira R, Bolt HM, Golka K. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2006. Acetaldehyde.
-
- Corbet JP, Mignani G. Selected patented cross-coupling reaction technologies. Chem Rev. 2006;106:2651–2710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

