Normal and abnormal development of the intrapericardial arterial trunks in humans and mice
- PMID: 22499773
- PMCID: PMC4228308
- DOI: 10.1093/cvr/cvs147
Normal and abnormal development of the intrapericardial arterial trunks in humans and mice
Abstract
Aims: The definitive cardiac outflow channels have three components: the intrapericardial arterial trunks; the arterial roots with valves; and the ventricular outflow tracts (OFTs). We studied the normal and abnormal development of the most distal of these, the arterial trunks, comparing findings in mice and humans.
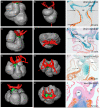
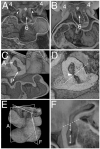
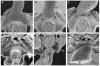
Methods and results: Using lineage tracing and three-dimensional visualization by episcopic reconstruction and scanning electron microscopy, we studied embryonic day 9.5-12.5 mouse hearts, clarifying the development of the OFTs distal to the primordia of the arterial valves. We characterize a transient aortopulmonary (AP) foramen, located between the leading edge of a protrusion from the dorsal wall of the aortic sac and the distal margins of the two outflow cushions. The foramen is closed by fusion of the protrusion, with its cap of neural crest cells (NCCs), with the NCC-filled cushions; the resulting structure then functioning transiently as an AP septum. Only subsequent to this closure is it possible to recognize, more proximally, the previously described AP septal complex. The adjacent walls of the intrapericardial trunks are derived from the protrusion and distal parts of the outflow cushions, whereas the lateral walls are formed from intrapericardial extensions of the pharyngeal mesenchyme derived from the second heart field.
Conclusions: We provide, for the first time, objective evidence of the mechanisms of closure of an AP foramen that exists distally between the lumens of the developing intrapericardial arterial trunks. Our findings provide insights into the formation of AP windows and the variants of common arterial trunk.
Figures



References
-
- Okamoto N, Akimoto N, Hidaka N, Shoji S, Sumida H. Formal genesis of the outflow tracts of the heart revisited: Previous works in the light of recent observations. Congenit Anom. 2010;59:141–158. - PubMed
-
- Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Am J Anat. 1942;71:343–370.
-
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. - PubMed
-
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1051. - PubMed
-
- Bartelings MM, Gittenberger-de Groot AC. The outflow tract of the heart-embryologic and morphologic correlations. Int J Cardiol. 1989;22:289–300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

