Present status and problems on molecular targeted therapy of cancer
- PMID: 22500155
- PMCID: PMC3322195
- DOI: 10.4143/crt.2012.44.1.1
Present status and problems on molecular targeted therapy of cancer
Abstract
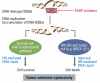
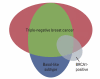
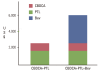
Numerous clinical trials of molecular targeted drugs for cancer have been conducted, with remarkable results for certain drugs and accumulation of "negative data" causing a hitch in the development plan for some other compounds. Five recent issues and problems of molecular targeted therapies were discussed critically. Drug discovery and effects against driver mutations (activating mutations) and problems: possibility for circumventing inherent and acquired resistance with the aim of achieving radical cure. Synthetic lethality: reasonable patient selection in individualized treatment strategy. Response rate and progression-free survival improvement with or without overall survival benefit and enhancement of toxicity in bevacizumab therapy: best endpoints for the evaluation of effect of antiangiogenic therapy. Negative data on small-molecule targeted therapy, primarily vascular endothelial growth factor tyrosine kinase inhibitors: loose GO or NO-GO decision criteria for further development of new compounds in early clinical trials. Effect of immunotherapy: difficulty to verify by proof of principle study. We are faced to many questions for the development of efficient personalized therapy. Accumulation of scientific global preclinical and clinical evidences is essential to use these new therapeutic modalities for the improvement of oncologic health care.
Keywords: Antiangiogenic therapy; Driver mutation; Endpoint determination; Molecular targeted therapy; Personalised therapy; Resistance; Synthetic letharity.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

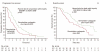
References
-
- Weinstein IB, Joe AK. Mechanisms of disease: oncogene addiction-a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–457. - PubMed
-
- Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Jänne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. - PMC - PubMed
-
- Chapman PB, Hauschild A, Robert C, Larkin JM, Haanen JB, Ribas A, et al. Phase III randomized open label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. J Clin Oncol. 2011;29(S):LBA4.
-
- CTV News: Vemurafenib in melanoma: was its phase 3 trial unethical? [Internet] Scarborough, ON: CTV Television Network; [cited 2011 Dec 20]. Available from: http://healthblog.ctv.ca.
LinkOut - more resources
Full Text Sources

