Glutathione homeostasis and functions: potential targets for medical interventions
- PMID: 22500213
- PMCID: PMC3303626
- DOI: 10.1155/2012/736837
Glutathione homeostasis and functions: potential targets for medical interventions
Abstract
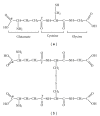
Glutathione (GSH) is a tripeptide, which has many biological roles including protection against reactive oxygen and nitrogen species. The primary goal of this paper is to characterize the principal mechanisms of the protective role of GSH against reactive species and electrophiles. The ancillary goals are to provide up-to-date knowledge of GSH biosynthesis, hydrolysis, and utilization; intracellular compartmentalization and interorgan transfer; elimination of endogenously produced toxicants; involvement in metal homeostasis; glutathione-related enzymes and their regulation; glutathionylation of sulfhydryls. Individual sections are devoted to the relationships between GSH homeostasis and pathologies as well as to developed research tools and pharmacological approaches to manipulating GSH levels. Special attention is paid to compounds mainly of a natural origin (phytochemicals) which affect GSH-related processes. The paper provides starting points for development of novel tools and provides a hypothesis for investigation of the physiology and biochemistry of glutathione with a focus on human and animal health.
Figures


References
-
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. A nuclear glutathione cycle within the cell cycle. Biochemical Journal. 2010;431(2):169–178. - PubMed
-
- Maher P. The effects of stress and aging on glutathione metabolism. Ageing Research Reviews. 2005;4(2):288–314. - PubMed
-
- Pócsi I, Prade RA, Penninckx MJ. Glutathione, altruistic metabolite in fungi. Advances in Microbial Physiology. 2004;49:1–76. - PubMed
-
- Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine. 1999;27(9-10):916–921. - PubMed
-
- Duan S, Chen C. S-nitrosylation/denitrosylation and apoptosis of immune cells. Cellular & Molecular Immunology. 2007;4(5):353–358. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

