Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy
- PMID: 22500508
- PMCID: PMC3329218
- DOI: 10.1016/j.trsl.2011.12.004
Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy
Abstract
Diabetic neuropathy is the most common and debilitating complication of diabetes mellitus with more than half of all patients developing altered sensation as a result of damage to peripheral sensory neurons. Hyperglycemia results in altered nerve conduction velocities, loss of epidermal innervation, and development of painful or painless signs and symptoms in the feet and hands. Current research has been unable to determine whether a patient will develop insensate or painful neuropathy or be protected from peripheral nerve damage all together. One mechanism that has been recognized to have a role in the pathogenesis of sensory neuron damage is the process of reactive dicarbonyls forming advanced glycation endproducts (AGEs) as a direct result of hyperglycemia. The glyoxalase system, composed of the enzymes glyoxalase I (GLO1) and glyoxalase II, is the main detoxification pathway involved in breaking down toxic reactive dicarbonyls before producing carbonyl stress and forming AGEs on proteins, lipids, or nucleic acids. This review discusses AGEs, GLO1, their role in diabetic neuropathy, and potential therapeutic targets of the AGE pathway.
Copyright © 2012 Mosby, Inc. All rights reserved.
Figures


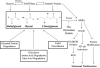
References
-
- Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011. In: Atlanta GA, editor. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011.
-
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. - PubMed
-
- Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007;3:331–40. - PubMed
-
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. - PubMed
-
- Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

