Wide-field spectral imaging of human ovary autofluorescence and oncologic diagnosis via previously collected probe data
- PMID: 22502561
- PMCID: PMC3380934
- DOI: 10.1117/1.JBO.17.3.036003
Wide-field spectral imaging of human ovary autofluorescence and oncologic diagnosis via previously collected probe data
Abstract
With no sufficient screening test for ovarian cancer, a method to evaluate the ovarian disease state quickly and nondestructively is needed. The authors have applied a wide-field spectral imager to freshly resected ovaries of 30 human patients in a study believed to be the first of its magnitude. Endogenous fluorescence was excited with 365-nm light and imaged in eight emission bands collectively covering the 400- to 640-nm range. Linear discriminant analysis was used to classify all image pixels and generate diagnostic maps of the ovaries. Training the classifier with previously collected single-point autofluorescence measurements of a spectroscopic probe enabled this novel classification. The process by which probe-collected spectra were transformed for comparison with imager spectra is described. Sensitivity of 100% and specificity of 51% were obtained in classifying normal and cancerous ovaries using autofluorescence data alone. Specificity increased to 69% when autofluorescence data were divided by green reflectance data to correct for spatial variation in tissue absorption properties. Benign neoplasm ovaries were also found to classify as nonmalignant using the same algorithm. Although applied ex vivo, the method described here appears useful for quick assessment of cancer presence in the human ovary.
© 2012 Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures

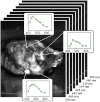





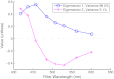

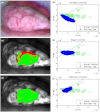
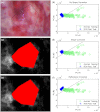

Similar articles
-
Parallel factor analysis of ovarian autofluorescence as a cancer diagnostic.Lasers Surg Med. 2012 Apr;44(4):282-95. doi: 10.1002/lsm.22014. Epub 2012 Mar 7. Lasers Surg Med. 2012. PMID: 22407572
-
Characterizing optical properties and spatial heterogeneity of human ovarian tissue using spatial frequency domain imaging.J Biomed Opt. 2016 Oct;21(10):101402. doi: 10.1117/1.JBO.21.10.101402. J Biomed Opt. 2016. PMID: 26822943 Free PMC article.
-
Autofluorescence of normal, benign, and malignant ovarian tissues: a pilot study.Photomed Laser Surg. 2009 Apr;27(2):325-35. doi: 10.1089/pho.2008.2261. Photomed Laser Surg. 2009. PMID: 18800945
-
Tryptophan autofluorescence imaging of neoplasms of the human colon.J Biomed Opt. 2012 Jan;17(1):016003. doi: 10.1117/1.JBO.17.1.016003. J Biomed Opt. 2012. PMID: 22352653
-
An overview of optical coherence tomography for ovarian tissue imaging and characterization.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Jan-Feb;7(1):1-16. doi: 10.1002/wnan.1306. Epub 2014 Oct 20. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25329515 Free PMC article. Review.
Cited by
-
An ultra-small nine-color spectrometer with a two-layer biparted ten-dichroic-mirror array and an image sensor.Sci Rep. 2022 Oct 3;12(1):16518. doi: 10.1038/s41598-022-20814-3. Sci Rep. 2022. PMID: 36192470 Free PMC article.
-
Ultraminiature optical design for multispectral fluorescence imaging endoscopes.J Biomed Opt. 2017 Mar 1;22(3):36013. doi: 10.1117/1.JBO.22.3.036013. J Biomed Opt. 2017. PMID: 28334332 Free PMC article.
-
Compact and ultracompact spectral imagers: technology and applications in biomedical imaging.J Biomed Opt. 2023 Apr;28(4):040901. doi: 10.1117/1.JBO.28.4.040901. Epub 2023 Apr 5. J Biomed Opt. 2023. PMID: 37035031 Free PMC article. Review.
-
Intraoperative multispectral and hyperspectral label-free imaging: A systematic review of in vivo clinical studies.J Biophotonics. 2019 Sep;12(9):e201800455. doi: 10.1002/jbio.201800455. Epub 2019 Apr 29. J Biophotonics. 2019. PMID: 30859757 Free PMC article.
-
Microscopy is better in color: development of a streamlined spectral light path for real-time multiplex fluorescence microscopy.Biomed Opt Express. 2022 Jun 7;13(7):3751-3772. doi: 10.1364/BOE.453657. eCollection 2022 Jul 1. Biomed Opt Express. 2022. PMID: 35991911 Free PMC article.
References
-
- Cancer Facts & Figures 2010, American Cancer Society, Atlanta (2010).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

