Instrument for fluorescence sensing of circulating cells with diffuse light in mice in vivo
- PMID: 22502573
- PMCID: PMC3380949
- DOI: 10.1117/1.JBO.17.3.037001
Instrument for fluorescence sensing of circulating cells with diffuse light in mice in vivo
Abstract
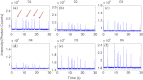
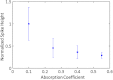
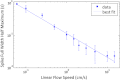
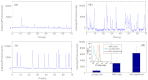
Accurate quantification of circulating cell populations in mice is important in many areas of preclinical biomedical research. Normally, this is done either by extraction and analysis of small blood samples or, more recently, by using microscopy-based in vivo fluorescence flow cytometry. We describe a new technological approach to this problem using detection of diffuse fluorescent light from relatively large blood vessels in vivo. The diffuse fluorescence flow cytometer (DFFC) uses a laser to illuminate a mouse limb and an array of optical fibers coupled to a high-sensitivity photomultiplier tube array operating in photon counting mode to detect weak fluorescence signals from cells. We first demonstrate that the DFFC instrument is capable of detecting fluorescent microspheres and Vybrant-DiD-labeled cells in a custom-made optical flow phantom with similar size, optical properties, linear flow rates, and autofluorescence as a mouse limb. We also present preliminary data demonstrating that the DFFC is capable of detecting circulating cells in nude mice in vivo. In principle, this device would allow interrogation of the whole blood volume of a mouse in minutes, with sensitivity improvement by several orders of magnitude compared to current approaches.
© 2012 Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures


References
-
- Bauernhofer T., et al. , “Association of disease progression and poor overall survival with detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer,” Oncol. Rep. 13(2), 179–184 (2005).OCRPEW - PubMed

