Mesenchymal-specific deletion of C/EBPβ suppresses pulmonary fibrosis
- PMID: 22503555
- PMCID: PMC3378850
- DOI: 10.1016/j.ajpath.2012.02.010
Mesenchymal-specific deletion of C/EBPβ suppresses pulmonary fibrosis
Abstract
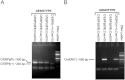
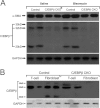
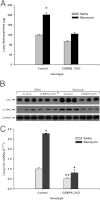
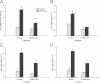
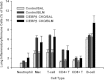
The CCAAT/enhancer-binding protein β (C/EBPβ) regulates a variety of factors and cellular responses associated with pulmonary fibrosis. To distinguish its role in the mesenchyme from that in other compartments, the effects of mesenchymal-specific deletion of C/EBPβ on pulmonary fibrosis was examined. Crossing of mice with the floxed C/EBPβ gene with α2(I) collagen enhancer-CreER(T)-bearing mice successfully generated progeny with a conditional knockout (CKO) of C/EBPβ in collagen I-expressing ("mesenchymal") cells only on treatment with tamoxifen (C/EBPβ CKO). When treated with an endotracheal bleomycin injection, C/EBPβ CKO mice showed significant attenuation of pulmonary fibrosis relative to control C/EBPβ-intact mice. C/EBPβ CKO mice also had reduced myofibroblasts in the lung. However, no significant differences in inflammatory/immune cell influx were noted in the mutant mice relative to the control mice. DNA microarray and real-time PCR analyses identified a series of myofibroblast differentiation regulators as novel target genes of C/EBPβ. Interestingly, C/EBPβ deficiency caused a marked induction of matrix metalloproteinase 12 expression, suggesting its potential role as a repressor, which could account for the noted reduction in fibrosis in the C/EBPβ-deficient mice. Thus, these findings indicate an essential role for C/EBPβ in the mesenchymal compartment in pulmonary fibrosis that is independent of its effects on inflammation or immune cell infiltration.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Phan S.H. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. - PubMed
-
- Skalli O., Schurch W., Seemayer T., Lagace R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989;60:275–285. - PubMed
-
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL52285/HL/NHLBI NIH HHS/United States
- R01 HL052285/HL/NHLBI NIH HHS/United States
- HL91775/HL/NHLBI NIH HHS/United States
- R01 HL077297/HL/NHLBI NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- HL28737/HL/NHLBI NIH HHS/United States
- HL77297/HL/NHLBI NIH HHS/United States
- P01 HL091775/HL/NHLBI NIH HHS/United States
- DK020572/DK/NIDDK NIH HHS/United States
- P01 HL031963/HL/NHLBI NIH HHS/United States
- R37 HL028737/HL/NHLBI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- HL31963/HL/NHLBI NIH HHS/United States
- R01 HL028737/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

