The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction
- PMID: 22503990
- PMCID: PMC3636677
- DOI: 10.1093/gerona/gls113
The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction
Abstract
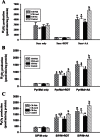
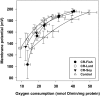
To investigate the role mitochondrial membrane lipids play in the actions of calorie restriction (CR), C57BL/6 mice were assigned to four groups (control and three 40% CR groups) and fed diets containing soybean oil (also in the control diet), fish oil, or lard. The fatty acid composition of the major mitochondrial phospholipid classes, proton leak, and H(2)O(2) production were measured in muscle mitochondria following 1 month of CR. The results indicate that phospholipid fatty acids reflected the polyunsaturated fatty acid profile of the dietary lipid sources. Capacity for Complex I- and III-linked H(2)O(2) production was decreased with CR, although there was no difference between CR groups. The CR lard group had lower proton leak than all other groups. The results indicate that a decreased degree of unsaturation in muscle mitochondrial membranes is not required for reduced H(2)O(2) production with CR. However, dietary lipids do have some influence on proton leak with CR.
Figures

Similar articles
-
The influence of dietary lipid composition on liver mitochondria from mice following 1 month of calorie restriction.Biosci Rep. 2012 Dec 13;33(1):83-95. doi: 10.1042/BSR20120060. Biosci Rep. 2012. PMID: 23098316 Free PMC article.
-
The influence of dietary lipid composition on skeletal muscle mitochondria from mice following eight months of calorie restriction.Physiol Res. 2014;63(1):57-71. doi: 10.33549/physiolres.932529. Epub 2013 Nov 1. Physiol Res. 2014. PMID: 24182343 Free PMC article.
-
The influence of dietary fat source on liver and skeletal muscle mitochondrial modifications and lifespan changes in calorie-restricted mice.Biogerontology. 2015 Oct;16(5):655-70. doi: 10.1007/s10522-015-9572-1. Epub 2015 Apr 10. Biogerontology. 2015. PMID: 25860863 Free PMC article. Review.
-
The Influence of Dietary Fat Source on Life Span in Calorie Restricted Mice.J Gerontol A Biol Sci Med Sci. 2015 Oct;70(10):1181-8. doi: 10.1093/gerona/glu177. Epub 2014 Oct 13. J Gerontol A Biol Sci Med Sci. 2015. PMID: 25313149 Free PMC article.
-
Dietary fats and membrane function: implications for metabolism and disease.Biol Rev Camb Philos Soc. 2005 Feb;80(1):155-69. doi: 10.1017/s1464793104006578. Biol Rev Camb Philos Soc. 2005. PMID: 15727042 Review.
Cited by
-
The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle.J Gerontol A Biol Sci Med Sci. 2019 May 16;74(6):760-769. doi: 10.1093/gerona/gly161. J Gerontol A Biol Sci Med Sci. 2019. PMID: 30010806 Free PMC article.
-
Dietary fat composition influences glomerular and proximal convoluted tubule cell structure and autophagic processes in kidneys from calorie-restricted mice.Aging Cell. 2016 Jun;15(3):477-87. doi: 10.1111/acel.12451. Epub 2016 Feb 8. Aging Cell. 2016. PMID: 26853994 Free PMC article.
-
Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene-disrupted mice.J Gerontol A Biol Sci Med Sci. 2013 Jun;68(6):639-51. doi: 10.1093/gerona/gls231. Epub 2012 Nov 29. J Gerontol A Biol Sci Med Sci. 2013. PMID: 23197187 Free PMC article.
-
The influence of dietary lipid composition on liver mitochondria from mice following 1 month of calorie restriction.Biosci Rep. 2012 Dec 13;33(1):83-95. doi: 10.1042/BSR20120060. Biosci Rep. 2012. PMID: 23098316 Free PMC article.
-
Mitochondrial ultrastructure and markers of dynamics in hepatocytes from aged, calorie restricted mice fed with different dietary fats.Exp Gerontol. 2014 Aug;56:77-88. doi: 10.1016/j.exger.2014.03.023. Epub 2014 Apr 4. Exp Gerontol. 2014. PMID: 24704714 Free PMC article.
References
-
- Yu BP. Membrane alteration as a basis of aging and the protective effects of calorie restriction. Mech Ageing Dev. 2005;126:1003–1010 - PubMed
-
- Pamplona R, Portero-Otin M, Riba D, et al. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res. 1998;39:1989–1994 - PubMed
-
- Portero-Otin M, Bellmunt MJ, Ruiz MC, Barja G, Pamplona R. Correlation of fatty acid unsaturation of the major liver mitochondrial phospholipid classes in mammals to their maximum life span potential. Lipids. 2001;36:491–498 - PubMed
-
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

